Origin and differentiation of vascular smooth muscle cells
- PMID: 25952975
- PMCID: PMC4532522
- DOI: 10.1113/JP270033
Origin and differentiation of vascular smooth muscle cells
Abstract
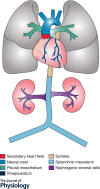
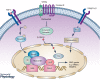
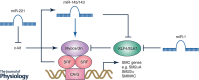
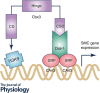
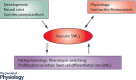
Vascular smooth muscle cells (SMCs), a major structural component of the vessel wall, not only play a key role in maintaining vascular structure but also perform various functions. During embryogenesis, SMC recruitment from their progenitors is an important step in the formation of the embryonic vascular system. SMCs in the arterial wall are mostly quiescent but can display a contractile phenotype in adults. Under pathophysiological conditions, i.e. vascular remodelling after endothelial dysfunction or damage, contractile SMCs found in the media switch to a secretory type, which will facilitate their ability to migrate to the intima and proliferate to contribute to neointimal lesions. However, recent evidence suggests that the mobilization and recruitment of abundant stem/progenitor cells present in the vessel wall are largely responsible for SMC accumulation in the intima during vascular remodelling such as neointimal hyperplasia and arteriosclerosis. Therefore, understanding the regulatory mechanisms that control SMC differentiation from vascular progenitors is essential for exploring therapeutic targets for potential clinical applications. In this article, we review the origin and differentiation of SMCs from stem/progenitor cells during cardiovascular development and in the adult, highlighting the environmental cues and signalling pathways that control phenotypic modulation within the vasculature.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
Similar articles
-
The transition of smooth muscle cells from a contractile to a migratory, phagocytic phenotype: direct demonstration of phenotypic modulation.J Physiol. 2016 Nov 1;594(21):6189-6209. doi: 10.1113/JP272729. Epub 2016 Aug 13. J Physiol. 2016. PMID: 27393389 Free PMC article.
-
Vascular Stem/Progenitor Cell Migration and Differentiation in Atherosclerosis.Antioxid Redox Signal. 2018 Jul 10;29(2):219-235. doi: 10.1089/ars.2017.7171. Epub 2017 Jul 5. Antioxid Redox Signal. 2018. PMID: 28537424 Review.
-
Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity.Novartis Found Symp. 2007;283:174-91; discussion 191-3, 238-41. doi: 10.1002/9780470319413.ch14. Novartis Found Symp. 2007. PMID: 18300422 Review.
-
Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function.Exp Hematol. 2010 Mar;38(3):246-257.e1. doi: 10.1016/j.exphem.2010.01.001. Epub 2010 Jan 11. Exp Hematol. 2010. PMID: 20067819 Free PMC article.
-
Macrophage-derived MMP-8 determines smooth muscle cell differentiation from adventitia stem/progenitor cells and promotes neointima hyperplasia.Cardiovasc Res. 2020 Jan 1;116(1):211-225. doi: 10.1093/cvr/cvz044. Cardiovasc Res. 2020. PMID: 30778537 Free PMC article.
Cited by
-
Induced pluripotent stem cell-derived vascular smooth muscle cells.Vasc Biol. 2019 Dec 12;2(1):R1-R15. doi: 10.1530/VB-19-0028. eCollection 2020. Vasc Biol. 2019. PMID: 32923972 Free PMC article. Review.
-
A Pilot Study of Subclinical Non-Capillary Peripapillary Perfusion Changes in Thyroid-Related Orbitopathy Detected Using Optical Coherence Tomography Angiography.Clin Ophthalmol. 2022 Mar 20;16:867-875. doi: 10.2147/OPTH.S356631. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35340669 Free PMC article.
-
miR-149-5p Inhibits Vascular Smooth Muscle Cells Proliferation, Invasion, and Migration by Targeting Histone Deacetylase 4 (HDAC4).Med Sci Monit. 2019 Oct 9;25:7581-7590. doi: 10.12659/MSM.916522. Med Sci Monit. 2019. PMID: 31595884 Free PMC article.
-
miR-137 and its target T-type CaV 3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway.Cell Prolif. 2020 Mar;53(3):e12774. doi: 10.1111/cpr.12774. Epub 2020 Feb 8. Cell Prolif. 2020. PMID: 32034930 Free PMC article.
-
Exercise Training Duration and Intensity Are Associated With Thicker Carotid Intima-Media Thickness but Improved Arterial Elasticity in Active Children and Adolescents.Front Cardiovasc Med. 2021 Jul 8;8:618294. doi: 10.3389/fcvm.2021.618294. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34307488 Free PMC article.
References
-
- Abedin M, Tintut Y. Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–676. - PubMed
-
- Aicher A, Zeiher AM. Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. - PubMed
-
- Alexander MR. Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. - PubMed
-
- Andreeva ER, Pugach IM. Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135:19–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

