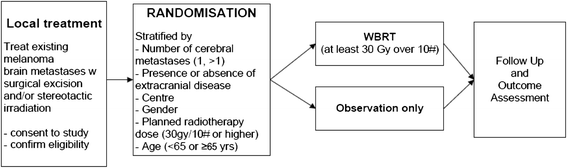
First interim analysis of a randomised trial of whole brain radiotherapy in melanoma brain metastases confirms high data quality
- PMID: 25952979
- PMCID: PMC4428505
- DOI: 10.1186/s13104-015-1153-5
First interim analysis of a randomised trial of whole brain radiotherapy in melanoma brain metastases confirms high data quality
Abstract
Background: Brain metastases are a common cause of death in patients with melanoma. The role of adjuvant whole brain radiotherapy (WBRT) following local treatment of intracranial melanoma metastases is controversial. The Australian and New Zealand Melanoma Trials Group (ANZMTG) and the Trans-Tasman Radiation Oncology Group (TROG) are leading the first ever single histology randomised trial investigating this question. The primary endpoint is distant intracranial failure on magnetic resonance imaging (MRI) within twelve months of randomisation. The first planned interim analysis was performed twelve months after randomisation of the 100(th) patient. The analysis was an opportunity to review completeness of the trial data to date.
Methods: All data received up to the end of twelve months after randomisation of the 100th patient was reviewed.
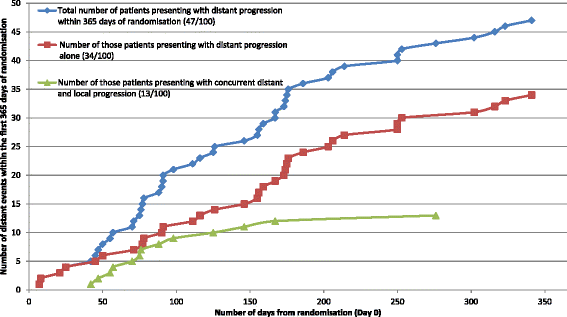
Results: Review of pathology reports confirmed that all 100 patients had stage IV melanoma and were appropriately entered into the study. Of the 47 distant intracranial events, 34 occurred in isolation (i.e. only distant failure was identified), whilst 13 were accompanied by local failure. Data review showed compliance with the protocol mandated MRI schedule and accuracy of intracranial failure reporting was very high. The Quality of Life (QoL) component of the study achieved a 91% completion rate. For the neurocognitive function (NCF) assessments, a high completion rate was maintained throughout the 12 month period. Where assessments were not performed at expected time points, valid reasons were noted. Radiotherapy quality was high. Of 50 patients who received WBRT, 32 were reviewed as per protocol design and there was only one major variation out of 308 data points reviewed (0.3%). There were minimal trial related adverse events (AEs) and no serious adverse events (SAEs). Pre-specified protocol stopping rules were not met.
Conclusions: The Data Safety Monitoring Committee (DSMC) recommended the trial continue recruitment after reviewing the unblinded data. The data provision and quality to date indicates that a reliable outcome will be obtained when the final analysis is performed. Accrual is ongoing with 156 out of 200 patients randomised to date (26(th) November 2014).
Figures
References
-
- De la Monte SM, Moore GW, Hutchins GM. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983;43:3427–33. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous