Transcription Factor ATAF1 in Arabidopsis Promotes Senescence by Direct Regulation of Key Chloroplast Maintenance and Senescence Transcriptional Cascades
- PMID: 25953103
- PMCID: PMC4741325
- DOI: 10.1104/pp.15.00567
Transcription Factor ATAF1 in Arabidopsis Promotes Senescence by Direct Regulation of Key Chloroplast Maintenance and Senescence Transcriptional Cascades
Abstract
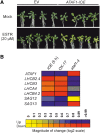
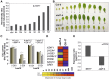
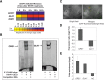
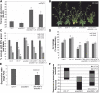
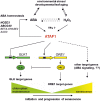
Senescence represents a fundamental process of late leaf development. Transcription factors (TFs) play an important role for expression reprogramming during senescence; however, the gene regulatory networks through which they exert their functions, and their physiological integration, are still largely unknown. Here, we identify the Arabidopsis (Arabidopsis thaliana) abscisic acid (ABA)- and hydrogen peroxide-activated TF Arabidopsis thaliana activating factor1 (ATAF1) as a novel upstream regulator of senescence. ATAF1 executes its physiological role by affecting both key chloroplast maintenance and senescence-promoting TFs, namely GOLDEN2-LIKE1 (GLK1) and ORESARA1 (Arabidopsis NAC092), respectively. Notably, while ATAF1 activates ORESARA1, it represses GLK1 expression by directly binding to their promoters, thereby generating a transcriptional output that shifts the physiological balance toward the progression of senescence. We furthermore demonstrate a key role of ATAF1 for ABA- and hydrogen peroxide-induced senescence, in accordance with a direct regulatory effect on ABA homeostasis genes, including nine-CIS-epoxycarotenoid dioxygenase3 involved in ABA biosynthesis and ABC transporter G family member40, encoding an ABA transport protein. Thus, ATAF1 serves as a core transcriptional activator of senescence by coupling stress-related signaling with photosynthesis- and senescence-related transcriptional cascades.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



Similar articles
-
ANAC032 Positively Regulates Age-Dependent and Stress-Induced Senescence in Arabidopsis thaliana.Plant Cell Physiol. 2016 Oct;57(10):2029-2046. doi: 10.1093/pcp/pcw120. Epub 2016 Jul 7. Plant Cell Physiol. 2016. PMID: 27388337
-
JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis.J Exp Bot. 2016 Feb;67(3):751-62. doi: 10.1093/jxb/erv487. Epub 2015 Nov 7. J Exp Bot. 2016. PMID: 26547795 Free PMC article.
-
Arabidopsis NAC016 promotes chlorophyll breakdown by directly upregulating STAYGREEN1 transcription.Plant Cell Rep. 2016 Jan;35(1):155-66. doi: 10.1007/s00299-015-1876-8. Epub 2015 Oct 6. Plant Cell Rep. 2016. PMID: 26441053
-
Specificity of H2O2 signaling in leaf senescence: is the ratio of H2O2 contents in different cellular compartments sensed in Arabidopsis plants?Cell Mol Biol Lett. 2022 Jan 6;27(1):4. doi: 10.1186/s11658-021-00300-w. Cell Mol Biol Lett. 2022. PMID: 34991444 Free PMC article. Review.
-
The pleiotropic functions of GOLDEN2-LIKE transcription factors in plants.Front Plant Sci. 2024 Aug 19;15:1445875. doi: 10.3389/fpls.2024.1445875. eCollection 2024. Front Plant Sci. 2024. PMID: 39224848 Free PMC article. Review.
Cited by
-
NAC Transcription Factor Family Regulation of Fruit Ripening and Quality: A Review.Cells. 2022 Feb 2;11(3):525. doi: 10.3390/cells11030525. Cells. 2022. PMID: 35159333 Free PMC article. Review.
-
Plant Nucleolar Stress Response, a New Face in the NAC-Dependent Cellular Stress Responses.Front Plant Sci. 2018 Jan 9;8:2247. doi: 10.3389/fpls.2017.02247. eCollection 2017. Front Plant Sci. 2018. PMID: 29375613 Free PMC article. Review.
-
CIRCADIAN CLOCK-ASSOCIATED 1 Inhibits Leaf Senescence in Arabidopsis.Front Plant Sci. 2018 Mar 6;9:280. doi: 10.3389/fpls.2018.00280. eCollection 2018. Front Plant Sci. 2018. PMID: 29559987 Free PMC article.
-
Genome-Wide Analysis of Alternative Splicing during Development and Drought Stress in Maize.Plant Physiol. 2016 Jan;170(1):586-99. doi: 10.1104/pp.15.01267. Epub 2015 Nov 18. Plant Physiol. 2016. PMID: 26582726 Free PMC article.
-
MORF2-mediated plastidial retrograde signaling is involved in stress response and skotomorphogenesis beyond RNA editing.Front Plant Sci. 2023 Mar 28;14:1146922. doi: 10.3389/fpls.2023.1146922. eCollection 2023. Front Plant Sci. 2023. PMID: 37056496 Free PMC article.
References
-
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

