The role of tau in the pathological process and clinical expression of Huntington's disease
- PMID: 25953777
- PMCID: PMC4572485
- DOI: 10.1093/brain/awv107
The role of tau in the pathological process and clinical expression of Huntington's disease
Abstract
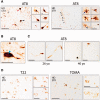
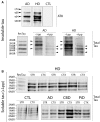
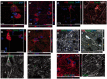
Huntington's disease is a neurodegenerative disorder caused by an abnormal CAG repeat expansion within exon 1 of the huntingtin gene HTT. While several genetic modifiers, distinct from the Huntington's disease locus itself, have been identified as being linked to the clinical expression and progression of Huntington's disease, the exact molecular mechanisms driving its pathogenic cascade and clinical features, especially the dementia, are not fully understood. Recently the microtubule associated protein tau, MAPT, which is associated with several neurodegenerative disorders, has been implicated in Huntington's disease. We explored this association in more detail at the neuropathological, genetic and clinical level. We first investigated tau pathology by looking for the presence of hyperphosphorylated tau aggregates, co-localization of tau with mutant HTT and its oligomeric intermediates in post-mortem brain samples from patients with Huntington's disease (n = 16) compared to cases with a known tauopathy and healthy controls. Next, we undertook a genotype-phenotype analysis of a large cohort of patients with Huntington's disease (n = 960) with a particular focus on cognitive decline. We report not only on the tau pathology in the Huntington's disease brain but also the association between genetic variation in tau gene and the clinical expression and progression of the disease. We found extensive pathological inclusions containing abnormally phosphorylated tau protein that co-localized in some instances with mutant HTT. We confirmed this related to the disease process rather than age, by showing it is also present in two patients with young-onset Huntington's disease (26 and 40 years old at death). In addition we demonstrate that tau oligomers (suggested to be the most likely neurotoxic tau entity) are present in the Huntington's disease brains. Finally we highlight the clinical significance of this pathology by demonstrating that the MAPT haplotypes affect the rate of cognitive decline in a large cohort of patients with Huntington's disease. Our findings therefore highlight a novel important role of tau in the pathogenic process and clinical expression of Huntington's disease, which in turn opens up new therapeutic avenues for this incurable condition.
Keywords: Huntington’s disease; dementia; neurofibrillary tangles; neuropathology; tau.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

Comment in
-
Neurodegenerative disease: Tau is linked to cognitive decline in Huntington disease.Nat Rev Neurol. 2015 Jun;11(6):310. doi: 10.1038/nrneurol.2015.87. Epub 2015 May 26. Nat Rev Neurol. 2015. PMID: 26008995 No abstract available.
References
-
- Anfossi M, Vuono R, Maletta R, Virdee K, Mirabelli M, Colao R, et al. Compound heterozygosity of 2 novel MAPT mutations in frontotemporal dementia. Neurobiol Aging. 2011;32:757, e1–e11. - PubMed
-
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–15. - PubMed
-
- Blum D, Herrera F, Francelle L, Mendes T, Basquin M, Obriot H, et al. Mutant huntingtin alters Tau phosphorylation and subcellular distribution. Hum Mol Genet. 2015;24:76–85. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

