Molecular controls of arterial morphogenesis
- PMID: 25953926
- PMCID: PMC4509635
- DOI: 10.1161/CIRCRESAHA.116.302953
Molecular controls of arterial morphogenesis
Abstract
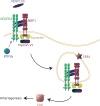
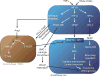
Formation of arterial vasculature, here termed arteriogenesis, is a central process in embryonic vascular development as well as in adult tissues. Although the process of capillary formation, angiogenesis, is relatively well understood, much remains to be learned about arteriogenesis. Recent discoveries point to the key role played by vascular endothelial growth factor receptor 2 in control of this process and to newly identified control circuits that dramatically influence its activity. The latter can present particularly attractive targets for a new class of therapeutic agents capable of activation of this signaling cascade in a ligand-independent manner, thereby promoting arteriogenesis in diseased tissues.
Keywords: angiogenesis factor; arteries; arteriogenesis; vascular endothelial growth factor A.
© 2015 American Heart Association, Inc.
Figures



References
-
- Morini MF, Dejana E. Transcriptional regulation of arterial differentiation via wnt, sox and notch. Current opinion in hematology. 2014 - PubMed
-
- Zheng X, Xu C, Smith AO, Stratman AN, Zou Z, Kleaveland B, Yuan L, Didiku C, Sen A, Liu X, Skuli N, Zaslavsky A, Chen M, Cheng L, Davis GE, Kahn ML. Dynamic regulation of the cerebral cavernous malformation pathway controls vascular stability and growth. Developmental cell. 2012;23:342–355. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

