Role of developmental factors in hypothalamic function
- PMID: 25954163
- PMCID: PMC4404869
- DOI: 10.3389/fnana.2015.00047
Role of developmental factors in hypothalamic function
Abstract
The hypothalamus is a brain region which regulates homeostasis by mediating endocrine, autonomic and behavioral functions. It is comprised of several nuclei containing distinct neuronal populations producing neuropeptides and neurotransmitters that regulate fundamental body functions including temperature and metabolic rate, thirst and hunger, sexual behavior and reproduction, circadian rhythm, and emotional responses. The identity, number and connectivity of these neuronal populations are established during the organism's development and are of crucial importance for normal hypothalamic function. Studies have suggested that developmental abnormalities in specific hypothalamic circuits can lead to obesity, sleep disorders, anxiety, depression and autism. At the molecular level, the development of the hypothalamus is regulated by transcription factors (TF), secreted growth factors, neuropeptides and their receptors. Recent studies in zebrafish and mouse have demonstrated that some of these molecules maintain their expression in the adult brain and subsequently play a role in the physiological functions that are regulated by hypothalamic neurons. Here, we summarize the involvement of some of the key developmental factors in hypothalamic development and function by focusing on the mouse and zebrafish genetic model organisms.
Keywords: Otp; PAC1; SF-1; SIM1; homeostasis; neuroendocrine; neuropeptides; zebrafish model system.
Figures
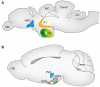
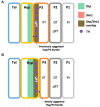

References
-
- Acampora D., Postiglione M. P., Avantaggiato V., Di Bonito M., Vaccarino F. M., Michaud J., et al. . (1999). Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 13, 2787–2800. 10.1101/gad.13.21.2787 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

