Analysis of medication and indication occurrences in clinical notes
- PMID: 25954414
- PMCID: PMC4419933
Analysis of medication and indication occurrences in clinical notes
Abstract
A medication indication is a valid reason to use medication. Comprehensive information on medication and its intended indications has valuable potential applications for patient treatments, quality improvements, and clinical decision support. Though there are some publicly available medication resources, this medication and indication information is comprised primarily of labeled uses approved by the FDA. Additionally, linking those medications and the corresponding indications is not easy to accomplish. Furthermore, research that analyzes actual medication and indication occurrences used in real clinical practice is limited. In this study, we compiled clinician-asserted medication and indication pairs from a large cohort of Mayo Clinic electronic medical records (EMRs) and normalized them to the standard forms (ie, medication to the RxNorm ingredient and indication to SNOMED-CT). We then analyzed medication and indication occurrences and compared them with the public resource in various ways, including off-label statistics.
Figures


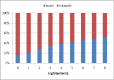
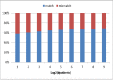
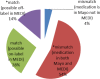
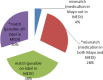
References
-
- Roth CP, Lim Y-W, Pevnick JM, Asch SM, McGlynn EA. The challenge of measuring quality of care from the electronic health record. American Journal of Medical Quality. 2009;24(5):385–394. - PubMed
-
- Tracy RP. ‘Deep phenotyping’: characterizing populations in the era of genomics and systems biology. Current opinion in lipidology. 2008;19(2):151–157. - PubMed
-
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Archives of Internal Medicine. 2006;166(9):1021–1026. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
