Equilibrium and kinetic studies of copper biosorption by dead Ceriporia lacerata biomass isolated from the litter of an invasive plant in China
- PMID: 25954509
- PMCID: PMC4423395
- DOI: 10.1186/s40201-015-0191-1
Equilibrium and kinetic studies of copper biosorption by dead Ceriporia lacerata biomass isolated from the litter of an invasive plant in China
Abstract
Background: Ceriporia lacerata, a strain of white-rot fungus isolated from the litter of an invasive plant (Solidago canadensis) in China, was little known about its properties and utilization. In this work, the copper(II) biosorption characteristics of formaldehyde inactivated C. lacerata biomass were examined as a function of initial pH, initial copper(II) concentration and contact time, and the adsorptive equilibrium and kinetics were simulated, too.
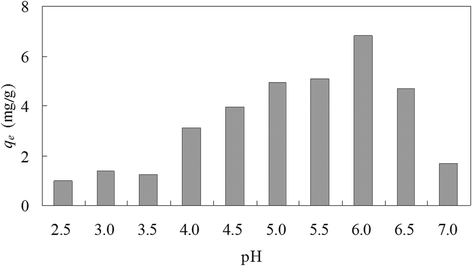
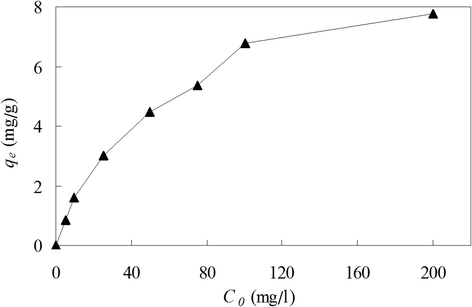
Results: The optimum pH was found to be 6.0 at experimental conditions of initial copper(II) concentration 100 mg/L, biomass dose 2 g/L, contact time 12 h, shaking rate 150 r/min and temperature 25°C. Biosorption equilibrium cost about 1 hour at experimental conditions of pH 6.0, initial copper(II) concentration 100 mg/L, C. lacerata dose 2 g/L, shaking rate 150 r/min and temperature 25°C. At optimum pH 6.0, highest copper(II) biosorption amounts were 6.79 and 7.76 mg/g for initial copper(II) concentration of 100 and 200 mg/L, respectively (with other experimental parameters of C. lacerata dose 2 g/L, shaking rate 150 r/min and temperature 25°C). The pseudo second-order adsorptive model gave the best adjustment for copper(II) biosorption kinetics. The equilibrium data fitted very well to both Langmuir and Freundlich adsorptive isotherm models.
Conclusions: Without further acid or alkali treatment for improving adsorption properties, formaldehyde inactivated C. lacerata biomass possesses good biosorption characteristics on copper(II) removal from aqueous solutions.
Keywords: Adsorption isotherm; Biosorption; Ceriporia lacerata; Copper; Kinetics.
Figures



References
-
- Çabuk A, Akar T, Tunali S, Gedikli S. Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomas of Pinus nigra: equilibrium and mechanism analysis. Chem Eng J. 2007;131(1–3):293–300. doi: 10.1016/j.cej.2006.12.011. - DOI
-
- Pümpel T, Schinner F. Native fungal pellets as a biosorbent for heavy metals. FEMS Microbiol Rev. 1993;11(1–3):159–64. doi: 10.1016/0168-6445(93)90037-A. - DOI
-
- Kapoor A, Viraraghavan T. Fungal biosorption - an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresource Technol. 1995;53(3):195–206.
LinkOut - more resources
Full Text Sources
Other Literature Sources

