Synthesis of the nakanishi ring-locked retinoid
- PMID: 25954523
- PMCID: PMC4411895
- DOI: 10.1155/2011/826792
Synthesis of the nakanishi ring-locked retinoid
Abstract
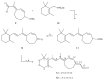
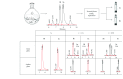
An optimized synthetic route to prepare ring-locked retinoid 1a has been developed. We fully describe a purification protocol that provides isomerically pure 1a in support of on-going proof of concept studies for the development of therapeutic agents to treat human ADRP. Additionally, we have found that isomerically pure 1a can be stored in amber vials under argon at -20°C for use over time (up to six months) without degradation. Thus, enabling 1a to be an accessible and valuable biological tool.
Figures



References
-
- Noorwez S. M., Kuksa V., Imanishi Y., et al. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. Journal of Biological Chemistry. 2003;278(16):14442–14450. doi: 10.1074/jbc.M300087200. - DOI - PMC - PubMed
-
- Zankel T., Ok H., Johnson R., et al. Bovine rhodopsin with 11-cis-locked retinal chromophore neither activates rhodopsin kinase nor undergoes conformational change upon irradiation. Journal of the American Chemical Society. 1990;112(13):5387–5388. doi: 10.1021/ja00169a077. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

