Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- PMID: 25954804
- PMCID: PMC4425567
- DOI: 10.1371/journal.ppat.1004897
Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
Abstract
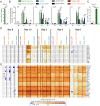
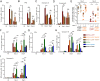
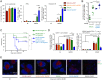
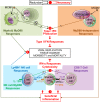
In mice, plasmacytoid dendritic cells (pDC) and natural killer (NK) cells both contribute to resistance to systemic infections with herpes viruses including mouse Cytomegalovirus (MCMV). pDCs are the major source of type I IFN (IFN-I) during MCMV infection. This response requires pDC-intrinsic MyD88-dependent signaling by Toll-Like Receptors 7 and 9. Provided that they express appropriate recognition receptors such as Ly49H, NK cells can directly sense and kill MCMV-infected cells. The loss of any one of these responses increases susceptibility to infection. However, the relative importance of these antiviral immune responses and how they are related remain unclear. In humans, while IFN-I responses are essential, MyD88 is dispensable for antiviral immunity. Hence, a higher redundancy has been proposed in the mechanisms promoting protective immune responses against systemic infections by herpes viruses during natural infections in humans. It has been assumed, but not proven, that mice fail to mount protective MyD88-independent IFN-I responses. In humans, the mechanism that compensates MyD88 deficiency has not been elucidated. To address these issues, we compared resistance to MCMV infection and immune responses between mouse strains deficient for MyD88, the IFN-I receptor and/or Ly49H. We show that selective depletion of pDC or genetic deficiencies for MyD88 or TLR9 drastically decreased production of IFN-I, but not the protective antiviral responses. Moreover, MyD88, but not IFN-I receptor, deficiency could largely be compensated by Ly49H-mediated antiviral NK cell responses. Thus, contrary to the current dogma but consistent with the situation in humans, we conclude that, in mice, in our experimental settings, MyD88 is redundant for IFN-I responses and overall defense against a systemic herpes virus infection. Moreover, we identified direct NK cell sensing of infected cells as one mechanism able to compensate for MyD88 deficiency in mice. Similar mechanisms likely contribute to protect MyD88- or IRAK4-deficient patients from viral infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. 10.1111/j.1600-065X.2008.00698.x - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

