A Metabotropic-Like Flux-Independent NMDA Receptor Regulates Ca2+ Exit from Endoplasmic Reticulum and Mitochondrial Membrane Potential in Cultured Astrocytes
- PMID: 25954808
- PMCID: PMC4425671
- DOI: 10.1371/journal.pone.0126314
A Metabotropic-Like Flux-Independent NMDA Receptor Regulates Ca2+ Exit from Endoplasmic Reticulum and Mitochondrial Membrane Potential in Cultured Astrocytes
Erratum in
-
Correction: A Metabotropic-Like Flux-Independent NMDA Receptor Regulates Ca2+ Exit from Endoplasmic Reticulum and Mitochondrial Membrane Potential in Cultured Astrocytes.PLoS One. 2018 Aug 20;13(8):e0202819. doi: 10.1371/journal.pone.0202819. eCollection 2018. PLoS One. 2018. PMID: 30125321 Free PMC article.
Abstract
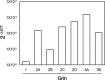
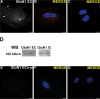
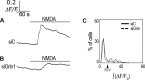
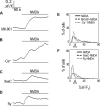
Astrocytes were long thought to be only structural cells in the CNS; however, their functional properties support their role in information processing and cognition. The ionotropic glutamate N-methyl D-aspartate (NMDA) receptor (NMDAR) is critical for CNS functions, but its expression and function in astrocytes is still a matter of research and debate. Here, we report immunofluorescence (IF) labeling in rat cultured cortical astrocytes (rCCA) of all NMDAR subunits, with phenotypes suggesting their intracellular transport, and their mRNA were detected by qRT-PCR. IF and Western Blot revealed GluN1 full-length synthesis, subunit critical for NMDAR assembly and transport, and its plasma membrane localization. Functionally, we found an iCa2+ rise after NMDA treatment in Fluo-4-AM labeled rCCA, an effect blocked by the NMDAR competitive inhibitors D(-)-2-amino-5-phosphonopentanoic acid (APV) and Kynurenic acid (KYNA) and dependent upon GluN1 expression as evidenced by siRNA knock down. Surprisingly, the iCa2+ rise was not blocked by MK-801, an NMDAR channel blocker, or by extracellular Ca2+ depletion, indicating flux-independent NMDAR function. In contrast, the IP3 receptor (IP3R) inhibitor XestosponginC did block this response, whereas a Ryanodine Receptor inhibitor did so only partially. Furthermore, tyrosine kinase inhibition with genistein enhanced the NMDA elicited iCa2+ rise to levels comparable to those reached by the gliotransmitter ATP, but with different population dynamics. Finally, NMDA depleted the rCCA mitochondrial membrane potential (mΔψ) measured with JC-1. Our results demonstrate that rCCA express NMDAR subunits which assemble into functional receptors that mediate a metabotropic-like, non-canonical, flux-independent iCa2+ increase.
Conflict of interest statement
Figures


Similar articles
-
NMDAR in cultured astrocytes: Flux-independent pH sensor and flux-dependent regulator of mitochondria and plasma membrane-mitochondria bridging.FASEB J. 2020 Dec;34(12):16622-16644. doi: 10.1096/fj.202001300R. Epub 2020 Nov 1. FASEB J. 2020. PMID: 33131132
-
Metabotropic glutamate receptor in C6BU-1 glioma cell has NMDA receptor-ion channel complex-like properties and interacts with serotonin2 receptor-stimulated signal transduction.J Neurochem. 1994 Oct;63(4):1346-53. doi: 10.1046/j.1471-4159.1994.63041346.x. J Neurochem. 1994. PMID: 7523590
-
Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro.J Neurosci. 2003 Apr 15;23(8):3364-72. doi: 10.1523/JNEUROSCI.23-08-03364.2003. J Neurosci. 2003. PMID: 12716944 Free PMC article.
-
Flux-Independent NMDAR Signaling: Molecular Mediators, Cellular Functions, and Complexities.Int J Mol Sci. 2018 Nov 29;19(12):3800. doi: 10.3390/ijms19123800. Int J Mol Sci. 2018. PMID: 30501045 Free PMC article. Review.
-
NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis.Int J Mol Sci. 2019 Jan 14;20(2):309. doi: 10.3390/ijms20020309. Int J Mol Sci. 2019. PMID: 30646531 Free PMC article. Review.
Cited by
-
Intracellular Ca2+ signalling: unexpected new roles for the usual suspect.Front Physiol. 2023 Jul 27;14:1210085. doi: 10.3389/fphys.2023.1210085. eCollection 2023. Front Physiol. 2023. PMID: 37576340 Free PMC article. Review.
-
The Role of NMDA Receptors in Alzheimer's Disease.Front Neurosci. 2019 Feb 8;13:43. doi: 10.3389/fnins.2019.00043. eCollection 2019. Front Neurosci. 2019. PMID: 30800052 Free PMC article. Review.
-
Two Signaling Modes Are Better than One: Flux-Independent Signaling by Ionotropic Glutamate Receptors Is Coming of Age.Biomedicines. 2024 Apr 16;12(4):880. doi: 10.3390/biomedicines12040880. Biomedicines. 2024. PMID: 38672234 Free PMC article. Review.
-
NMDA Receptors in Astrocytes.Neurochem Res. 2020 Jan;45(1):122-133. doi: 10.1007/s11064-019-02750-3. Epub 2019 Feb 14. Neurochem Res. 2020. PMID: 30767094 Review.
-
Reactive Transformation and Increased BDNF Signaling by Hippocampal Astrocytes in Response to MK-801.PLoS One. 2015 Dec 23;10(12):e0145651. doi: 10.1371/journal.pone.0145651. eCollection 2015. PLoS One. 2015. PMID: 26700309 Free PMC article.
References
-
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–3. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

