Force-sensitive autoinhibition of the von Willebrand factor is mediated by interdomain interactions
- PMID: 25954888
- PMCID: PMC4423058
- DOI: 10.1016/j.bpj.2015.03.041
Force-sensitive autoinhibition of the von Willebrand factor is mediated by interdomain interactions
Abstract
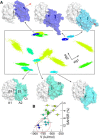
Von Willebrand factor (VWF) plays a central role in hemostasis. Triggered by shear-stress, it adheres to platelets at sites of vascular injury. Inactivation of VWF has been associated to the shielding of its adhesion sites and proteolytic cleavage. However, the molecular nature of this shielding and its coupling to cleavage under shear-forces in flowing blood remain unknown. In this study, we describe, to our knowledge, a new force-sensory mechanism for VWF-platelet binding, which addresses these questions, based on a combination of molecular dynamics (MD) simulations, atomic force microscopy (AFM), and microfluidic experiments. Our MD simulations demonstrate that the VWF A2 domain targets a specific region at the VWF A1 domain, corresponding to the binding site of the platelet glycoprotein Ibα (GPIbα) receptor, thereby causing its blockage. This implies autoinhibition of the VWF for the binding of platelets mediated by the A1-A2 protein-protein interaction. During force-probe MD simulations, a stretching force dissociated the A1A2 complex, thereby unblocking the GPIbα binding site. Dissociation was found to be coupled to the unfolding of the A2 domain, with dissociation predominantly occurring before exposure of the cleavage site in A2, an observation that is supported by our AFM experiments. This suggests that the A2 domain prevents platelet binding in a force-dependent manner, ensuring that VWF initiates hemostasis before inactivation by proteolytic cleavage. Microfluidic experiments with an A2-deletion VWF mutant resulted in increased platelet binding, corroborating the key autoinhibitory role of the A2 domain within VWF multimers. Overall, autoinhibition of VWF mediated by force-dependent interdomain interactions offers the molecular basis for the shear-sensitive growth of VWF-platelet aggregates, and might be similarly involved in shear-induced VWF self-aggregation and other force-sensing functions in hemostasis.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Mutual A domain interactions in the force sensing protein von Willebrand factor.J Struct Biol. 2017 Jan;197(1):57-64. doi: 10.1016/j.jsb.2016.04.012. Epub 2016 Apr 23. J Struct Biol. 2017. PMID: 27113902
-
Purified A2 domain of von Willebrand factor binds to the active conformation of von Willebrand factor and blocks the interaction with platelet glycoprotein Ibalpha.J Thromb Haemost. 2007 Jul;5(7):1363-70. doi: 10.1111/j.1538-7836.2007.02536.x. Epub 2007 Mar 27. J Thromb Haemost. 2007. PMID: 17389010
-
The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibα.J Biol Chem. 2013 Nov 8;288(45):32289-32301. doi: 10.1074/jbc.M113.504001. Epub 2013 Sep 23. J Biol Chem. 2013. PMID: 24062306 Free PMC article. Clinical Trial.
-
Biophysical approaches promote advances in the understanding of von Willebrand factor processing and function.Adv Biol Regul. 2017 Jan;63:81-91. doi: 10.1016/j.jbior.2016.09.010. Epub 2016 Sep 28. Adv Biol Regul. 2017. PMID: 27717713 Review.
-
A biophysical view on von Willebrand factor activation.J Cell Physiol. 2018 Feb;233(2):799-810. doi: 10.1002/jcp.25887. Epub 2017 May 16. J Cell Physiol. 2018. PMID: 28256724 Review.
Cited by
-
Coarse-Grain Modeling of Shear-Induced Binding between von Willebrand Factor and Collagen.Biophys J. 2018 Apr 24;114(8):1816-1829. doi: 10.1016/j.bpj.2018.02.017. Biophys J. 2018. PMID: 29694861 Free PMC article.
-
Long-ranged Protein-glycan Interactions Stabilize von Willebrand Factor A2 Domain from Mechanical Unfolding.Sci Rep. 2018 Oct 30;8(1):16017. doi: 10.1038/s41598-018-34374-y. Sci Rep. 2018. PMID: 30375453 Free PMC article.
-
Exposure of Von Willebrand Factor Cleavage Site in A1A2A3-Fragment under Extreme Hydrodynamic Shear.Polymers (Basel). 2021 Nov 12;13(22):3912. doi: 10.3390/polym13223912. Polymers (Basel). 2021. PMID: 34833213 Free PMC article.
-
Intradimer forces and their implication for conformations of von Willebrand factor multimers.Biophys J. 2021 Mar 2;120(5):899-911. doi: 10.1016/j.bpj.2021.01.022. Epub 2021 Jan 30. Biophys J. 2021. PMID: 33524374 Free PMC article.
-
Diverse activities of von Willebrand factor in traumatic brain injury and associated coagulopathy.J Thromb Haemost. 2020 Dec;18(12):3154-3162. doi: 10.1111/jth.15096. Epub 2020 Oct 6. J Thromb Haemost. 2020. PMID: 32931638 Free PMC article. Review.
References
-
- Schneppenheim R., Budde U. von Willebrand factor: the complex molecular genetics of a multidomain and multifunctional protein. J. Thromb. Haemost. 2011;9(Suppl. 1):209–215. - PubMed
-
- Chen H., Fallah M.A., Alexander-Katz A. Blood-clotting-inspired reversible polymer—colloid composite assembly in flow. Nat. Commun. 2013;4:1333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

