Chasing the Origin of Viruses: Capsid-Forming Genes as a Life-Saving Preadaptation within a Community of Early Replicators
- PMID: 25955384
- PMCID: PMC4425637
- DOI: 10.1371/journal.pone.0126094
Chasing the Origin of Viruses: Capsid-Forming Genes as a Life-Saving Preadaptation within a Community of Early Replicators
Abstract
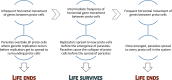
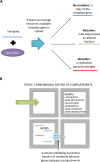
Virus capsids mediate the transfer of viral genetic information from one cell to another, thus the origin of the first viruses arguably coincides with the origin of the viral capsid. Capsid genes are evolutionarily ancient and their emergence potentially predated even the origin of first free-living cells. But does the origin of the capsid coincide with the origin of viruses, or is it possible that capsid-like functionalities emerged before the appearance of true viral entities? We set to investigate this question by using a computational simulator comprising primitive replicators and replication parasites within a compartment matrix. We observe that systems with no horizontal gene transfer between compartments collapse due to the rapidly emerging replication parasites. However, introduction of capsid-like genes that induce the movement of randomly selected genes from one compartment to another rescues life by providing the non-parasitic replicators a mean to escape their current compartments before the emergence of replication parasites. Capsid-forming genes can mediate the establishment of a stable meta-population where parasites cause only local tragedies but cannot overtake the whole community. The long-term survival of replicators is dependent on the frequency of horizontal transfer events, as systems with either too much or too little genetic exchange are doomed to succumb to replication-parasites. This study provides a possible scenario for explaining the origin of viral capsids before the emergence of genuine viruses: in the absence of other means of horizontal gene transfer between compartments, evolution of capsid-like functionalities may have been necessary for early life to prevail.
Conflict of interest statement
Figures



Similar articles
-
Virus world as an evolutionary network of viruses and capsidless selfish elements.Microbiol Mol Biol Rev. 2014 Jun;78(2):278-303. doi: 10.1128/MMBR.00049-13. Microbiol Mol Biol Rev. 2014. PMID: 24847023 Free PMC article. Review.
-
Origin of viruses: primordial replicators recruiting capsids from hosts.Nat Rev Microbiol. 2019 Jul;17(7):449-458. doi: 10.1038/s41579-019-0205-6. Nat Rev Microbiol. 2019. PMID: 31142823 Review.
-
The logic of virus evolution.Cell Host Microbe. 2022 Jul 13;30(7):917-929. doi: 10.1016/j.chom.2022.06.008. Cell Host Microbe. 2022. PMID: 35834963 Review.
-
The ancient Virus World and evolution of cells.Biol Direct. 2006 Sep 19;1:29. doi: 10.1186/1745-6150-1-29. Biol Direct. 2006. PMID: 16984643 Free PMC article.
-
The place of viruses in biology in light of the metabolism- versus-replication-first debate.Hist Philos Life Sci. 2012;34(3):391-406. Hist Philos Life Sci. 2012. PMID: 23316568
Cited by
-
The hidden life of integrative and conjugative elements.FEMS Microbiol Rev. 2017 Jul 1;41(4):512-537. doi: 10.1093/femsre/fux008. FEMS Microbiol Rev. 2017. PMID: 28369623 Free PMC article. Review.
-
The Double-Stranded DNA Virosphere as a Modular Hierarchical Network of Gene Sharing.mBio. 2016 Aug 2;7(4):e00978-16. doi: 10.1128/mBio.00978-16. mBio. 2016. PMID: 27486193 Free PMC article.
-
Viruses and mobile elements as drivers of evolutionary transitions.Philos Trans R Soc Lond B Biol Sci. 2016 Aug 19;371(1701):20150442. doi: 10.1098/rstb.2015.0442. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27431520 Free PMC article. Review.
-
Evolution of an archaeal virus nucleocapsid protein from the CRISPR-associated Cas4 nuclease.Biol Direct. 2015 Oct 29;10:65. doi: 10.1186/s13062-015-0093-2. Biol Direct. 2015. PMID: 26514828 Free PMC article.
-
Multiple origins of viral capsid proteins from cellular ancestors.Proc Natl Acad Sci U S A. 2017 Mar 21;114(12):E2401-E2410. doi: 10.1073/pnas.1621061114. Epub 2017 Mar 6. Proc Natl Acad Sci U S A. 2017. PMID: 28265094 Free PMC article.
References
-
- Pawlowski A, Rissanen I, Bamford JK, Krupovic M, Jalasvuori M. Gammasphaerolipovirus, a newly proposed bacteriophage genus, unifies viruses of halophilic archaea and thermophilic bacteria within the novel family Sphaerolipoviridae . Arch Virol. 2014;159: 1541–1554. 10.1007/s00705-013-1970-6 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

