Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels
- PMID: 25955826
- PMCID: PMC4425559
- DOI: 10.1371/journal.pone.0125503
Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels
Abstract
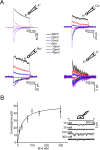
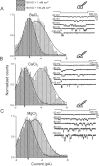
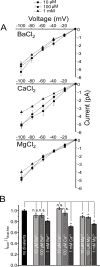
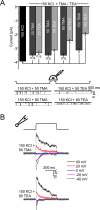
Members of the eukaryotic PIEZO family (the human orthologs are noted hPIEZO1 and hPIEZO2) form cation-selective mechanically-gated channels. We characterized the selectivity of human PIEZO1 (hPIEZO1) for alkali ions: K+, Na+, Cs+ and Li+; organic cations: TMA and TEA, and divalents: Ba2+, Ca2+, Mg2+ and Mn2+. All monovalent ions permeated the channel. At a membrane potential of -100 mV, Cs+, Na+ and K+ had chord conductances in the range of 35-55 pS with the exception of Li+, which had a significantly lower conductance of ~ 23 pS. The divalents decreased the single-channel permeability of K+, presumably because the divalents permeated slowly and occupied the open channel for a significant fraction of the time. In cell-attached mode, 90 mM extracellular divalents had a conductance for inward currents carried by the divalents of: 25 pS for Ba2+ and 15 pS for Ca2+ at -80 mV and 10 pS for Mg2+ at -50 mV. The organic cations, TMA and TEA, permeated slowly and attenuated K+ currents much like the divalents. As expected, the channel K+ conductance increased with K+ concentration saturating at ~ 45 pS and the KD of K+ for the channel was 32 mM. Pure divalent ion currents were of lower amplitude than those with alkali ions and the channel opening rate was lower in the presence of divalents than in the presence of monovalents. Exposing cells to the actin disrupting reagent cytochalasin D increased the frequency of openings in cell-attached patches probably by reducing mechanoprotection.
Conflict of interest statement
Figures



References
-
- Hille B. Ion channels of Excitable Membranes (Sinauer Associates, Inc; ). 2001:441–70.
-
- Ding S, Sachs F. Ion permeation and block of P2X(2) purinoceptors: single channel recordings. The Journal of membrane biology. 1999;172(3):215–23. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

