Automated Solution-Phase Synthesis of β-1,4-Mannuronate and β-1,4-Mannan
- PMID: 25955886
- PMCID: PMC4460921
- DOI: 10.1021/acs.orglett.5b01013
Automated Solution-Phase Synthesis of β-1,4-Mannuronate and β-1,4-Mannan
Abstract
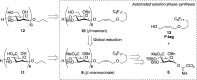
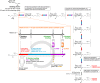
The first automated solution-phase synthesis of β-1,4-mannuronate and β-1,4-mannan oligomers has been accomplished by using a β-directing C-5 carboxylate strategy. By utilizing fluorous-tag assisting purification after repeated reaction cycles, β-1,4-mannuronate was synthesized up to a hexasaccharide with limited loading of a glycosyl donor (up to 3.5 equiv) for each glycosylation cycle due to the homogeneous solution-phase reaction condition. After a global reduction of the uronates, the β-1,4-mannan hexasaccharide was obtained, thereby demonstrating a new approach to β-mannan synthesis.
Figures


References
-
- Heuckendorff M.; Bendix J.; Pedersen C. M.; Bols M. Org. Lett. 2014, 16, 1116–1119. - PubMed
- Baek J. Y.; Lee B.-Y.; Jo M. G.; Kim K. S. J. Am. Chem. Soc. 2009, 131, 17705–17713. - PubMed
- Stork G.; La Clair J. J. J. Am. Chem. Soc. 1996, 118, 247–248.
- Wu X.; Bundle D. R. J. Org. Chem. 2005, 70, 7381–7388. - PubMed
-
- Walters K. R. Jr.; Serianni A. S.; Sformo T.; Barnes B. M.; Duman J. G. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 20210–20215. - PMC - PubMed
- Ishiwata A.; Sakurai A.; Nishimiya Y.; Tsuda S.; Ito Y. J. Am. Chem. Soc. 2011, 133, 19524–19535. - PubMed
- Crich D.; Rahaman M. Y. J. Org. Chem. 2011, 76, 8611–8620. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

