Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives
- PMID: 25955889
- PMCID: PMC6272754
- DOI: 10.3390/molecules20058125
Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives
Abstract
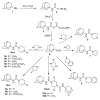
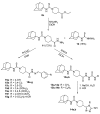
The reaction of 1-adamantyl isothiocyanate 4 with the various cyclic secondary amines yielded the corresponding N-(1-adamantyl)carbothioamides 5a-e, 6, 7, 8a-c and 9. Similarly, the reaction of 4 with piperazine and trans-2,5-dimethylpiperazine in 2:1 molar ratio yielded the corresponding N,N'-bis(1-adamantyl)piperazine-1,4-dicarbothioamides 10a and 10b, respectively. The reaction of N-(1-adamantyl)-4-ethoxycarbonylpiperidine-1-carbothioamide 8c with excess hydrazine hydrate yielded the target carbohydrazide 11, in addition to 4-(1-adamantyl)thiosemicarbazide 12 as a minor product. The reaction of the carbohydrazide 11 with methyl or phenyl isothiocyanate followed by heating in aqueous sodium hydroxide yielded the 1,2,4-triazole analogues 14a and 14b. The reaction of the carbohydrazide 11 with various aromatic aldehydes yielded the corresponding N'-arylideneamino derivatives 15a-g. The compounds 5a-e, 6, 7, 8a-c, 9, 10a, 10b, 14a, 14b and 15a-g were tested for in vitro antimicrobial activity against certain strains of pathogenic Gram-positive and Gram-negative bacteria and the yeast-like fungus Candida albicans. The compounds 5c, 5d, 5e, 6, 7, 10a, 10b, 15a, 15f and 15g showed potent antibacterial activity against one or more of the tested microorganisms. The oral hypoglycemic activity of compounds 5c, 6, 8b, 9, 14a and 15b was determined in streptozotocin (STZ)-induced diabetic rats. Compound 5c produced significant reduction of serum glucose levels, compared to gliclazide.
Conflict of interest statement
The authors declare no conflicts of interest in this study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous