Chemical Safety Assessment Using Read-Across: Assessing the Use of Novel Testing Methods to Strengthen the Evidence Base for Decision Making
- PMID: 25956009
- PMCID: PMC4671246
- DOI: 10.1289/ehp.1409342
Chemical Safety Assessment Using Read-Across: Assessing the Use of Novel Testing Methods to Strengthen the Evidence Base for Decision Making
Abstract
Background: Safety assessment for repeated dose toxicity is one of the largest challenges in the process to replace animal testing. This is also one of the proof of concept ambitions of SEURAT-1, the largest ever European Union research initiative on alternative testing, co-funded by the European Commission and Cosmetics Europe. This review is based on the discussion and outcome of a workshop organized on initiative of the SEURAT-1 consortium joined by a group of international experts with complementary knowledge to further develop traditional read-across and include new approach data.
Objectives: The aim of the suggested strategy for chemical read-across is to show how a traditional read-across based on structural similarities between source and target substance can be strengthened with additional evidence from new approach data--for example, information from in vitro molecular screening, "-omics" assays and computational models--to reach regulatory acceptance.
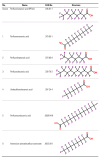
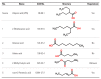
Methods: We identified four read-across scenarios that cover typical human health assessment situations. For each such decision context, we suggested several chemical groups as examples to prove when read-across between group members is possible, considering both chemical and biological similarities.
Conclusions: We agreed to carry out the complete read-across exercise for at least one chemical category per read-across scenario in the context of SEURAT-1, and the results of this exercise will be completed and presented by the end of the research initiative in December 2015.
Conflict of interest statement
The contents of this paper are the views of the authors and do not necessarily represent the views and policies of the European Commission, the European Chemicals Agency (ECHA) and the European Food Safety Authority (EFSA).
A multidisciplinary workshop expert panel wrote this review. The workshop was chaired by K.B. with support of E.B.
Cosmetics Europe–The Personal Care Association is an industrial European trade association co-financing the SEURAT-1 research initiative. P.A. was employed by Cosmetics Europe. T.S. has been employed since October 2015 under a consultant contract with Cosmetics Europe. K.B. and C.M. (Procter & Gamble), F.G. (L’Oréal), D.K. (Henkel), and A.W. (Unilever) are all employed by companies acting as Active Corporate Members of Cosmetics Europe. E.C. was employed by The Dow Chemical Company, Midland, MI, USA.The other authors declare they have no actual or potential competing financial interests.
Figures


References
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. - PubMed
-
- Ball N, Bartels M, Budinsky R, Klapacz J, Hays S, Kirman C, et al. The challenge of using read-across within the EU REACH regulatory framework; how much uncertainty is too much? Dipropylene glycol methyl ether acetate, an exemplary case study. Regul Toxicol Pharmacol. 2014;68:212–221. - PubMed
-
- Berggren E. SEURAT-1 proof-of-concepts. In: Towards the Replacement of in Vivo Repeated Dose Systemic Toxicity Testing. Vol. 4 (Gocht T, Schwarz M, eds). Le Perreux-sur-Marne, France:Coach Consortium, 86–93. 2014. Available: http://www.seurat-1.eu/media/download_gallery/SEURAT-1_Annual%20Report_V... [accessed 24 September 2014]
-
- Blackburn K, Bjerke D, Daston G, Felter S, Mahony C, Naciff J, et al. Case studies to test: a framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regul Toxicol Pharmacol. 2011;60:120–135. - PubMed
-
- Blackburn K, Stuard SB. A framework to facilitate consistent characterization of read across uncertainty. Regul Toxicol Pharmacol. 2014;68:353–362. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
