Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy
- PMID: 25956030
- PMCID: PMC4484219
- DOI: 10.1016/j.ajpath.2015.03.007
Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy
Abstract
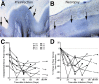
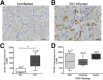
HIV-associated sensory neuropathy remains the most common neurological complication of HIV infection and is characterized by dorsal root ganglion (DRG) inflammation and intraepidermal nerve fiber density (IENFD) loss. Chronic peripheral immune cell activation and accumulation may cause damage to the DRG, but has not been fully investigated yet. By using an SIV-infected, CD8-lymphocyte-depleted rhesus macaque model, we defined immune cells surrounding DRG neurons and their role in DRG pathology, measured cell traffic from the bone marrow to the DRGs using 5-bromo-2-deoxyuridine (BrdU) pulse, and serially measured IENFD. We found an increase in CD68(+) and CD163(+) macrophages in DRGs of SIV-infected animals. MAC387(+) recently recruited monocytes/macrophages were increased, along with BrdU(+) cells, in the DRGs of SIV-infected macaques. We demonstrated that 78.1% of all BrdU(+) cells in DRGs were also MAC387(+). The number of BrdU(+) monocytes correlated with severe DRG histopathology, which included neuronophagia, neuronal loss, and Nageotte nodules. These data demonstrate that newly recruited MAC387(+)BrdU(+) macrophages may play a significant role in DRG pathogenesis. IENFD decreased early (day 21), consistent with the development of sensory neuropathy in SIV-infected macaques. Decreased IENFD was associated with elevated BrdU(+) cells in the DRG. These data suggest that increased recruitment of macrophages to DRG is associated with severe DRG histopathology and IENFD loss.
Copyright © 2015 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Ellis R.J., Rosario D., Clifford D.B., McArthur J.C., Simpson D., Alexander T., Gelman B.B., Vaida F., Collier A., Marra C.M., Ances B., Atkinson J.H., Dworkin R.H., Morgello S., Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. - PMC - PubMed
-
- Nicholas P.K., Mauceri L., Slate Ciampa A., Corless I.B., Raymond N., Barry D.J., Viamonte Ros A. Distal sensory polyneuropathy in the context of HIV/AIDS. J Assoc Nurses AIDS Care. 2007;18:32–40. - PubMed
-
- Verma S., Estanislao L., Simpson D. HIV-associated neuropathic pain: epidemiology, pathophysiology and management. CNS Drugs. 2005;19:325–334. - PubMed
-
- Ellis R.J., Marquie-Beck J., Delaney P., Alexander T., Clifford D.B., McArthur J.C., Simpson D.M., Ake C., Collier A.C., Gelman B.B., McCutchan J.A., Morgello S., Grant I. Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol. 2008;64:566–572. - PMC - PubMed
-
- Pardo C.A., McArthur J.C., Griffin J.W. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

