A Review of Clinical Translation of Inorganic Nanoparticles
- PMID: 25956384
- PMCID: PMC4540735
- DOI: 10.1208/s12248-015-9780-2
A Review of Clinical Translation of Inorganic Nanoparticles
Abstract
Inorganic nanoparticles are widely used for therapeutic and diagnostic purposes as they offer unique features as compared with their organic and polymeric counterparts. As such, inorganic nanoparticles represent an exciting opportunity to develop drug delivery and imaging systems that are poised to tackle unique challenges which are currently unaddressed in clinical settings. Despite these clear advantages, very few inorganic nanoparticle systems have entered the clinic. Here, we review the current clinical landscape of inorganic nanoparticle systems and their opportunities and challenges, with particular emphasis on gold-, iron-oxide- and silica-based nanoparticle systems. Key examples of inorganic nanoparticles that are currently being investigated in the clinic (e.g., trials which are recruiting or currently active but not completed) are highlighted, along with the preclinical work that these examples have leveraged to transition from the lab to the clinic.
Figures
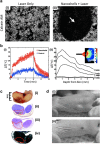


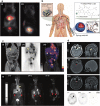
References
-
- Na HB, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009;21(21):2133–48. doi: 10.1002/adma.200802366. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

