Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit(POS)/NKX2.5(POS) bone marrow stem cells: implication in stem cell translational medicine
- PMID: 25956503
- PMCID: PMC4446115
- DOI: 10.1186/s13287-015-0085-2
Activation of Notch1 signalling promotes multi-lineage differentiation of c-Kit(POS)/NKX2.5(POS) bone marrow stem cells: implication in stem cell translational medicine
Abstract
Introduction: Transplantation of bone marrow mesenchymal stem cells (BMSCs) can repair injured hearts. However, whether BMSC populations contain cells with cardiac stem cell characteristics is ill-defined. We report here that Notch signalling can promote differentiation of c-Kit(POS)/NKX2.5(POS) BMSCs into cardiomyocyte-like cells.
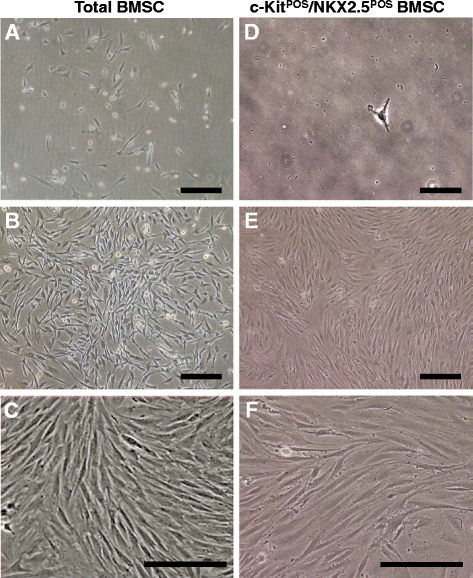
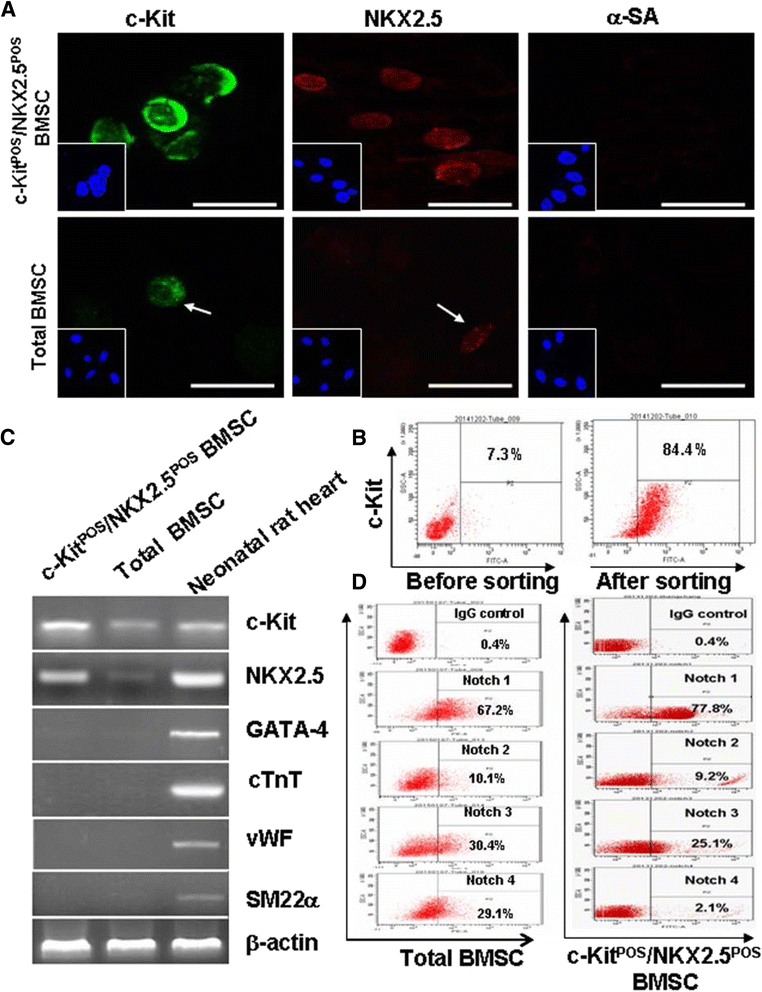
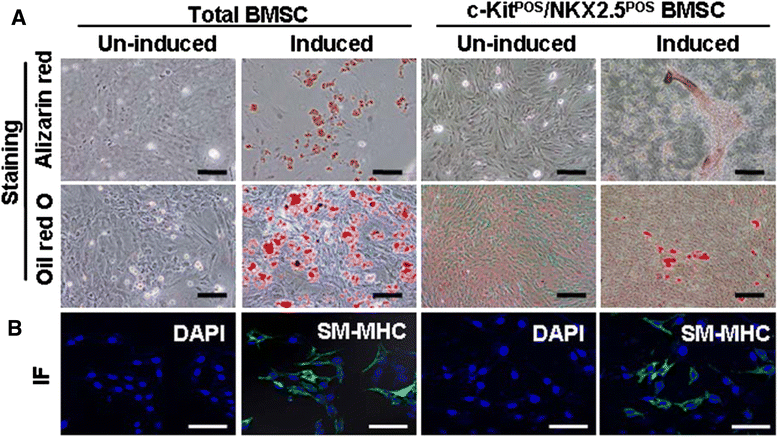
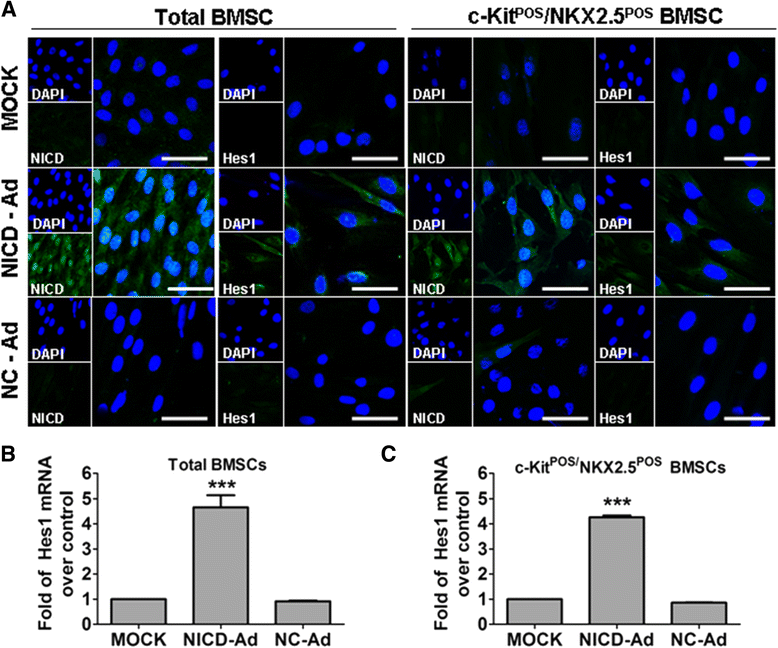
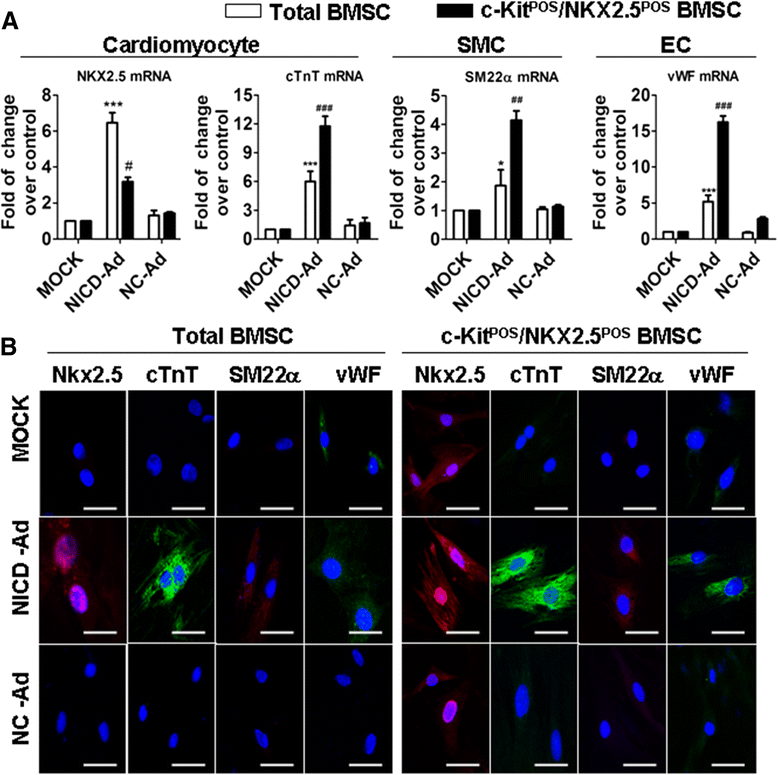
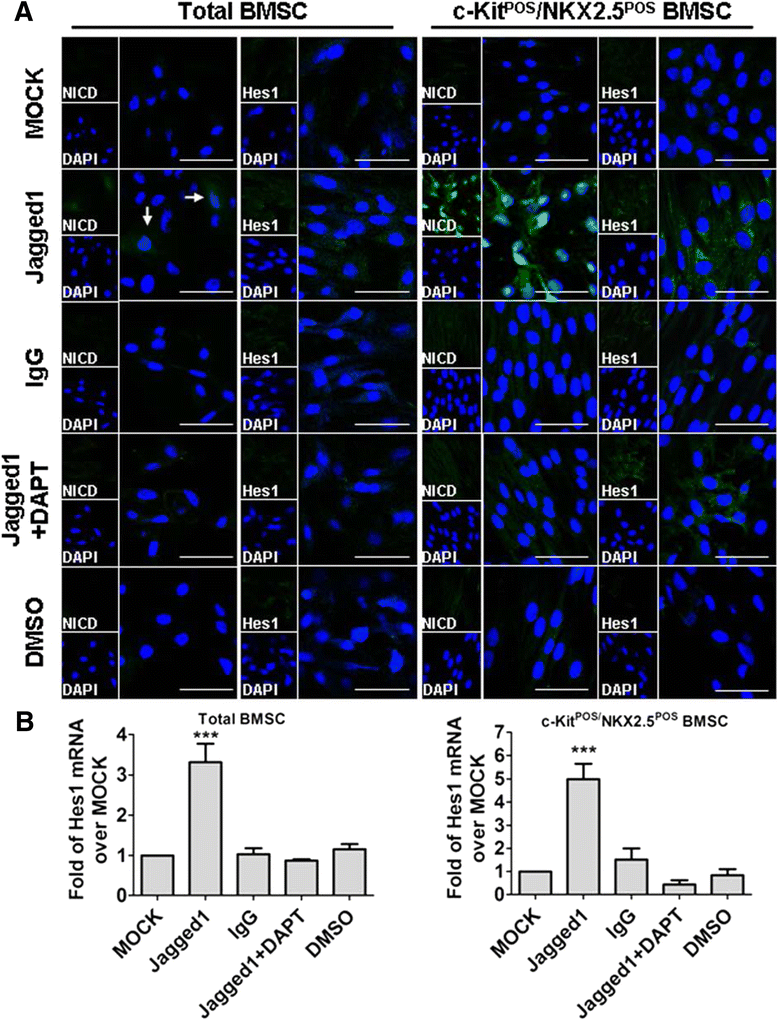
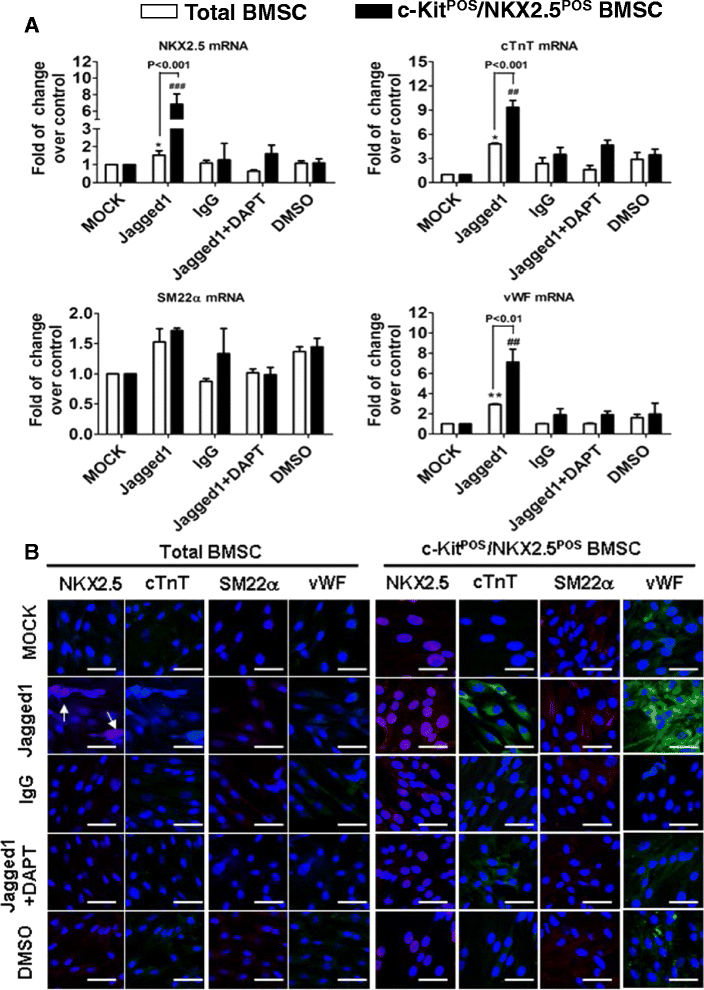
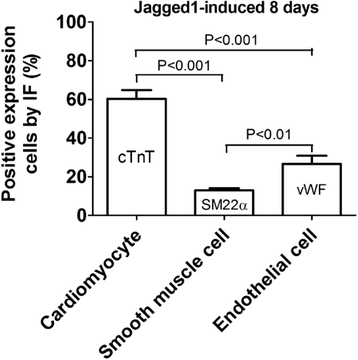
Methods: Total BMSCs were isolated from Sprague-Dawley rat femurs and c-Kit(POS) cells were purified. c-Kit(POS)/NKX2.5(POS) cells were isolated by single-cell cloning, and the presence of cardiomyocyte, smooth muscle cell (SMC), and endothelial cell differentiation markers assessed by immunofluorescence staining and semi-quantitative reverse-transcription polymerase chain reaction (RT-PCR) analysis. Levels of c-Kit and Notch1-4 in total BMSCs and c-Kit(POS)/NKX2.5(POS) BMSCs were quantitated by flow cytometry. Following infection with an adenovirus over-expressing Notch1 intracellular domain (NICD), total BMSCs and c-Kit(POS)/NKX2.5(POS) cells were assessed for differentiation to cardiomyocyte, SMC, and endothelial cell lineages by immunofluorescence staining and real-time quantitative RT-PCR. Total BMSCs and c-Kit(POS)/NKX2.5(POS) cells were treated with the Notch1 ligand Jagged1 and markers of cardiomyocyte, SMC, and endothelial cell differentiation were examined by immunofluorescence staining and real-time quantitative RT-PCR analysis.
Results: c-Kit(POS)/NKX2.5(POS) cells were present among total BMSC populations, and these cells did not express markers of adult cardiomyocyte, SMC, or endothelial cell lineages. c-Kit(POS)/NKX2.5(POS) BMSCs exhibited a multi-lineage differentiation potential similar to total BMSCs. Following sorting, the c-Kit level in c-Kit(POS)/NKX2.5(POS) BMSCs was 84.4%. Flow cytometry revealed that Notch1 was the predominant Notch receptor present in total BMSCs and c-Kit(POS)/NKX2.5(POS) BMSCs. Total BMSCs and c-Kit(POS)/NKX2.5(POS) BMSCs overexpressing NICD had active Notch1 signalling accompanied by differentiation into cardiomyocyte, SMC, and endothelial cell lineages. Treatment of total BMSCs and c-Kit(POS)/NKX2.5(POS) BMSCs with exogenous Jagged1 activated Notch1 signalling and drove multi-lineage differentiation, with a tendency towards cardiac lineage differentiation in c-Kit(POS)/NKX2.5(POS) BMSCs.
Conclusions: c-Kit(POS)/NKX2.5(POS) cells exist in total BMSC pools. Activation of Notch1 signalling contributed to multi-lineage differentiation of c-Kit(POS)/NKX2.5(POS) BMSCs, favouring differentiation into cardiomyocytes. These findings suggest that modulation of Notch1 signalling may have potential utility in stem cell translational medicine.
Figures
Similar articles
-
Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes.Biochem Biophys Res Commun. 2006 Mar 10;341(2):320-5. doi: 10.1016/j.bbrc.2005.12.182. Epub 2006 Jan 10. Biochem Biophys Res Commun. 2006. PMID: 16413496
-
Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration.J Mol Cell Cardiol. 2011 Sep;51(3):399-408. doi: 10.1016/j.yjmcc.2011.06.004. Epub 2011 Jun 13. J Mol Cell Cardiol. 2011. PMID: 21712044
-
[The expression of GATA-4 and Nkx2.5 gene in the transformation of Rattus mesenchymal stem cells into cardiomyocytes].Sichuan Da Xue Xue Bao Yi Xue Ban. 2008 Nov;39(6):882-5. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008. PMID: 19253817 Chinese.
-
"String theory" of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results.Circ Res. 2015 Mar 27;116(7):1216-30. doi: 10.1161/CIRCRESAHA.116.305557. Circ Res. 2015. PMID: 25814683 Free PMC article. Review.
-
Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction.Trends Cardiovasc Med. 2010 Oct;20(7):228-31. doi: 10.1016/j.tcm.2011.11.006. Trends Cardiovasc Med. 2010. PMID: 22293023 Free PMC article. Review.
Cited by
-
The cardiac niche role in cardiomyocyte differentiation of rat bone marrow-derived stromal cells: comparison between static and microfluidic cell culture methods.EXCLI J. 2018 Aug 1;17:762-774. doi: 10.17179/excli2018-1539. eCollection 2018. EXCLI J. 2018. PMID: 30190666 Free PMC article.
-
Inhibition of Notch Signaling Promotes the Differentiation of Epicardial Progenitor Cells into Adipocytes.Stem Cells Int. 2021 Apr 9;2021:8859071. doi: 10.1155/2021/8859071. eCollection 2021. Stem Cells Int. 2021. PMID: 33897781 Free PMC article.
-
A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction.Stem Cell Res Ther. 2017 Nov 2;8(1):242. doi: 10.1186/s13287-017-0697-9. Stem Cell Res Ther. 2017. PMID: 29096705 Free PMC article. Review.
-
Mechanisms supporting potential use of bone marrow-derived mesenchymal stem cells in psychocardiology.Am J Transl Res. 2019 Nov 15;11(11):6717-6738. eCollection 2019. Am J Transl Res. 2019. PMID: 31814884 Free PMC article. Review.
-
Thioredoxin-1 Protects Bone Marrow-Derived Mesenchymal Stromal Cells from Hyperoxia-Induced Injury In Vitro.Oxid Med Cell Longev. 2018 Jan 21;2018:1023025. doi: 10.1155/2018/1023025. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29599892 Free PMC article.
References
-
- Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. - DOI - PMC - PubMed
-
- Surder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–79. doi: 10.1161/CIRCULATIONAHA.112.001035. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

