Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer
- PMID: 25956750
- PMCID: PMC4445286
- DOI: 10.1186/s12885-015-1406-7
Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer
Abstract
Background: The aim of the trial was to compare two active adjuvant chemotherapy regimens in patients with early stage colorectal cancer (CRC).
Methods: Patients were assigned to oxaliplatin, leucovorin and 5-FU for 12 cycles (group A, FOLFOX6) or oxaliplatin and capecitabine for eight cycles (group B, CAPOX). Primary endpoint was disease-free survival (DFS). Tumors were classified as mismatch repair proficient (pMMR) or deficient (dMMR) according to MLH1, PMS2, MSH2 and MSH6 protein expression. KRAS exon two and BRAF V600E mutational status were also assessed.
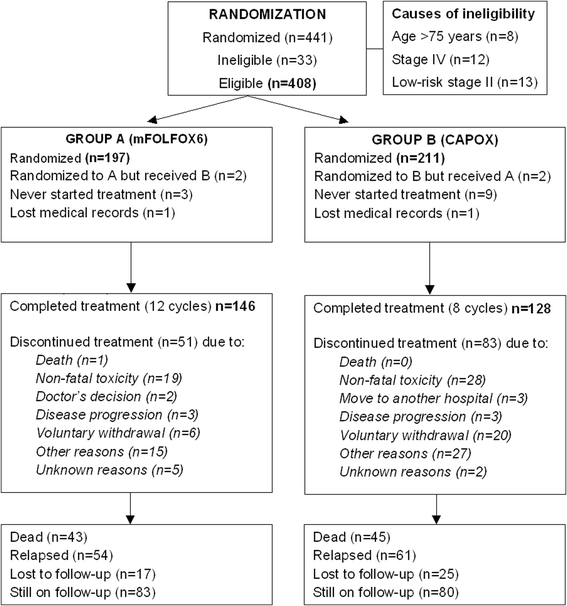
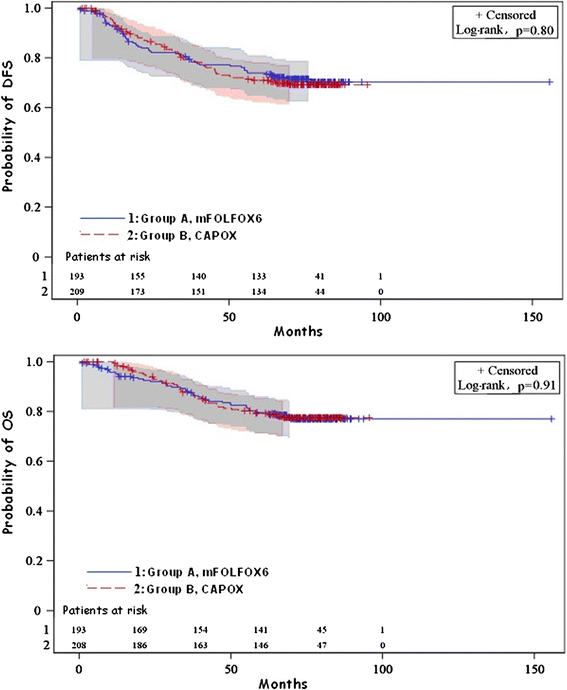
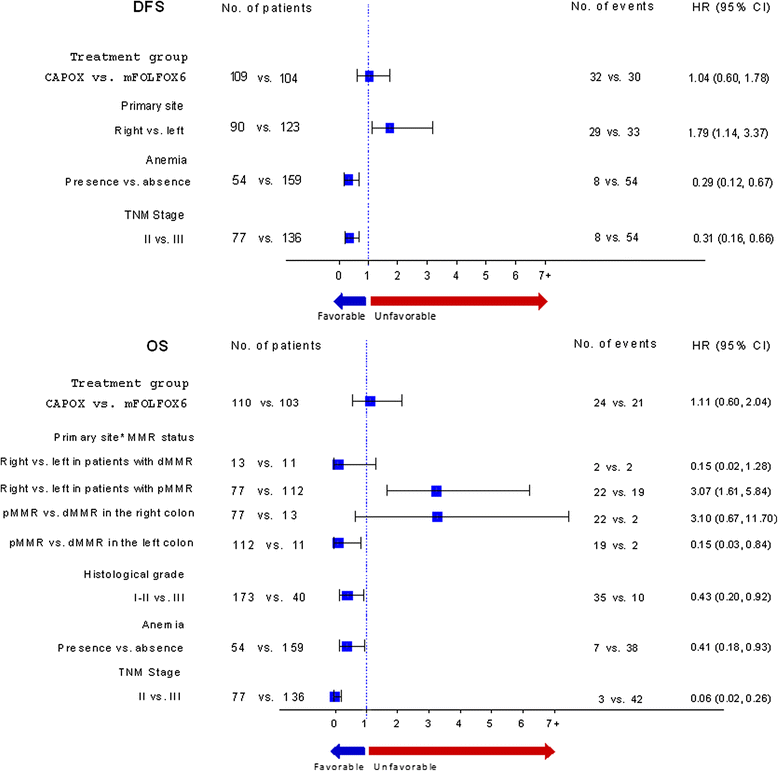
Results: Between 2005 and 2008, 441 patients were enrolled, with 408 patients being eligible. After a median follow-up of 74.7 months, 3-year DFS was 79.8 % (95 % CI 76.5-83.4) in the FOLFOX group and 79.5 % (95 % CI 75.9-83.1) in the CAPOX group (p = 0.78). Three-year OS was 87.2 % (95 % CI 84.1-91.1) in the FOLFOX and 86.9 % (95 % CI 83.4-89.9) in the CAPOX group (p = 0.84). Among 306 available tumors, 11.0 % were dMMR, 34.0 % KRAS mutant and 4.9 % BRAF mutant. Multivariate analysis showed that primary site in the left colon, earlier TNM stage and the presence of anemia at diagnosis were associated with better DFS and overall survival (OS), while grade one-two tumors were associated with better OS. Finally, a statistically significant interaction was detected between the primary site and MMR status (p = 0.010), while KRAS mutated tumors were associated with shorter DFS. However, the sample was too small for safe conclusions.
Conclusions: No significant differences were observed in the efficacy of FOLFOX versus CAPOX as adjuvant treatment in high-risk stage II or stage III CRC patients, but definitive conclusions cannot be drawn because of the small sample size.
Trial registration: ANZCTR 12610000509066 . Date of Registration: June 21, 2010.
Figures
References
-
- Midgley RS, Kerr DJ. Systematic adjuvant chemotherapy for colorectal cancer. In: Bleiberg H, Rougier P, Wilke HJ, editors. Management of colorectal cancer. London: Martin Dunitz; 1998. pp. 126–37.
-
- Colon cancer. Clinical practice guidelines in oncology (NCCN guidelines). http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

