Biochemical and Kinetic Characterization of the Eukaryotic Phosphotransacetylase Class IIa Enzyme from Phytophthora ramorum
- PMID: 25956919
- PMCID: PMC4486679
- DOI: 10.1128/EC.00007-15
Biochemical and Kinetic Characterization of the Eukaryotic Phosphotransacetylase Class IIa Enzyme from Phytophthora ramorum
Abstract
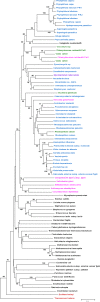
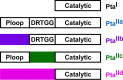
Phosphotransacetylase (Pta), a key enzyme in bacterial metabolism, catalyzes the reversible transfer of an acetyl group from acetyl phosphate to coenzyme A (CoA) to produce acetyl-CoA and Pi. Two classes of Pta have been identified based on the absence (Pta(I)) or presence (Pta(II)) of an N-terminal regulatory domain. Pta(I) has been fairly well studied in bacteria and one genus of archaea; however, only the Escherichia coli and Salmonella enterica Pta(II) enzymes have been biochemically characterized, and they are allosterically regulated. Here, we describe the first biochemical and kinetic characterization of a eukaryotic Pta from the oomycete Phytophthora ramorum. The two Ptas from P. ramorum, designated PrPta(II)1 and PrPta(II)2, both belong to class II. PrPta(II)1 displayed positive cooperativity for both acetyl phosphate and CoA and is allosterically regulated. We compared the effects of different metabolites on PrPta(II)1 and the S. enterica Pta(II) and found that, although the N-terminal regulatory domains share only 19% identity, both enzymes are inhibited by ATP, NADP, NADH, phosphoenolpyruvate (PEP), and pyruvate in the acetyl-CoA/Pi-forming direction but are differentially regulated by AMP. Phylogenetic analysis of bacterial, archaeal, and eukaryotic sequences identified four subtypes of Pta(II) based on the presence or absence of the P-loop and DRTGG subdomains within the N-terminal regulatory domain. Although the E. coli, S. enterica, and P. ramorum enzymes all belong to the IIa subclass, our kinetic analysis has indicated that enzymes within a subclass can still display differences in their allosteric regulation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Bubb WA, Wright LC, Cagney M, Santangelo RT, Sorrell TC, Kuchel PW. 1999. Heteronuclear NMR studies of metabolites produced by Cryptococcus neoformans in culture media: identification of possible virulence factors. Magn Reson Med 42:442–453. - PubMed
-
- Oura E. 1977. Reaction products of yeast fermentations. Process Biochem 12:19–21.
-
- Mazet M, Morand P, Biran M, Bouyssou G, Courtois P, Daulouede S, Millerioux Y, Franconi JM, Vincendeau P, Moreau P, Bringaud F. 2013. Revisiting the central metabolism of the bloodstream forms of Trypanosoma brucei: production of acetate in the mitochondrion is essential for parasite viability. PLoS Negl Trop Dis 7:e2587. doi:10.1371/journal.pntd.0002587. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

