Hypoxia is an effective stimulus for vesicular release of ATP from human umbilical vein endothelial cells
- PMID: 25956988
- PMCID: PMC4502406
- DOI: 10.1016/j.placenta.2015.04.005
Hypoxia is an effective stimulus for vesicular release of ATP from human umbilical vein endothelial cells
Abstract
Introduction: Hypoxia induces dilatation of the umbilical vein by releasing autocoids from endothelium; prostaglandins (PGs), adenosine and nitric oxide (NO) have been implicated. ATP is vasoactive, thus we tested whether hypoxia releases ATP from primary Human Umbilical Vein Endothelial Cells (HUVEC).
Methods: HUVEC were grown on inserts under no-flow conditions. ATP was assayed by luciferin-luciferase and visualised by quinacrine labeling. Intracellular Ca(2+) ([Ca(2+)]i) was imaged with Fura-2.
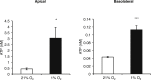
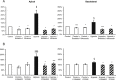
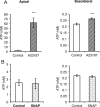
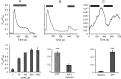
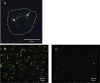
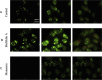
Results: ATP release occurred constitutively and was increased by hypoxia (PO2: 150-8 mmHg), ∼10-fold more from apical, than basolateral surface. Constitutive ATP release was decreased, while hypoxia-induced release was abolished by brefeldin or monensin A, inhibitors of vesicular transport, and LY294002 or Y27632, inhibitors of phosphoinositide 3-kinases (PI3K) and Rho-associated protein kinase (ROCK). ATP release was unaffected by NO donor, but increased by calcium ionophore, by >60-fold from apical, but <25% from basolateral surface. Hypoxia induced a small increase in [Ca(2+)]i compared with ATP (10 μM); hypoxia inhibited the ATP response. Quinacrine-ATP fluorescent loci in the perinuclear space, were diminished by hypoxia and monensin, whereas brefeldin A increased fluorescence intensity, consistent with inhibition of anterograde transport.
Discussion: Hypoxia within the physiological range releases ATP from HUVEC, particularly from apical/adluminal surfaces by exocytosis, via an increase in [Ca(2+)]i, PI3K and ROCK, independently of NO. We propose that hypoxia releases ATP at concentrations sufficient to induce umbilical vein dilation via PGs and NO and improve fetal blood flow, but curbs amplification of ATP release by autocrine actions of ATP, so limiting its pro-inflammatory effects.
Keywords: ATP; Exocytosis; HUVEC; Hypoxia; Vesicles.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells.Am J Physiol Lung Cell Mol Physiol. 2009 Nov;297(5):L954-64. doi: 10.1152/ajplung.00038.2009. Epub 2009 Aug 14. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19684203 Free PMC article.
-
Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular.J Cardiovasc Pharmacol. 2001 Dec;38(6):900-8. doi: 10.1097/00005344-200112000-00012. J Cardiovasc Pharmacol. 2001. PMID: 11707694
-
Hypoxia stimulates vesicular ATP release from rat osteoblasts.J Cell Physiol. 2009 Jul;220(1):155-62. doi: 10.1002/jcp.21745. J Cell Physiol. 2009. PMID: 19259945
-
Extracellular ATP attenuates ischemia-induced caspase-3 cleavage in human endothelial cells.Biochem Biophys Res Commun. 2012 Aug 24;425(2):230-6. doi: 10.1016/j.bbrc.2012.07.073. Epub 2012 Jul 22. Biochem Biophys Res Commun. 2012. PMID: 22828512
-
[ATP release pathways in vascular endothelial cells].Nihon Yakurigaku Zasshi. 2004 Jun;123(6):403-11. doi: 10.1254/fpj.123.403. Nihon Yakurigaku Zasshi. 2004. PMID: 15170080 Review. Japanese.
Cited by
-
Purinergic signalling in the cardiovascular system-a tribute to Geoffrey Burnstock.Purinergic Signal. 2021 Mar;17(1):63-69. doi: 10.1007/s11302-020-09734-x. Epub 2020 Nov 5. Purinergic Signal. 2021. PMID: 33151503 Free PMC article.
-
Mitochondrial targeting as a novel therapy for stroke.Brain Circ. 2018 Jul-Sep;4(3):84-94. doi: 10.4103/bc.bc_14_18. Epub 2018 Oct 9. Brain Circ. 2018. PMID: 30450413 Free PMC article. Review.
-
Fueling recovery: The importance of energy coupling between angiogenesis and osteogenesis during fracture healing.Bone Rep. 2024 Mar 25;21:101757. doi: 10.1016/j.bonr.2024.101757. eCollection 2024 Jun. Bone Rep. 2024. PMID: 38577251 Free PMC article. Review.
-
Enhancing immunotherapy with tumour-responsive nanomaterials.Nat Rev Clin Oncol. 2025 Apr;22(4):262-282. doi: 10.1038/s41571-025-01000-6. Epub 2025 Mar 6. Nat Rev Clin Oncol. 2025. PMID: 40050505 Review.
-
Spiking Neural Network with Linear Computational Complexity for Waveform Analysis in Amperometry.Sensors (Basel). 2021 May 10;21(9):3276. doi: 10.3390/s21093276. Sensors (Basel). 2021. PMID: 34068538 Free PMC article.
References
-
- Boura A.L.A., Walters W.A.W., Read M.A., Leitch I.M. Autacoids and control of human placental blood flow. Clin Exp Pharmacol Physiol. 1994;21:737–748. - PubMed
-
- Mildenberger E., Siegel G., Versmold H.T. Oxygen-dependent regulation of membrane potential and vascular tone of human umbilical vein. Am J Obstet Gynecol. 1999;181:696–700. - PubMed
-
- Mildenberger E., Biesel B., Siegel G., Versmold H.T. Nitric oxide and endothelin in oxygen-dependent regulation of vascular tone of human umbilical vein. Am J Physiol Heart Circ Physiol. 2003;285:H1730–H1737. - PubMed
-
- Spaans F., de Vos P., Bakker W.W., van Goor H., Faas M.M. Danger signals from ATP and adenosine in pregnancy and preeclampsia. Hypertension. 2014;63:1154–1160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

