Schwann Cells: Development and Role in Nerve Repair
- PMID: 25957303
- PMCID: PMC4484967
- DOI: 10.1101/cshperspect.a020487
Schwann Cells: Development and Role in Nerve Repair
Abstract
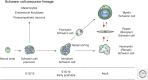
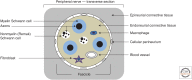
Schwann cells develop from the neural crest in a well-defined sequence of events. This involves the formation of the Schwann cell precursor and immature Schwann cells, followed by the generation of the myelin and nonmyelin (Remak) cells of mature nerves. This review describes the signals that control the embryonic phase of this process and the organogenesis of peripheral nerves. We also discuss the phenotypic plasticity retained by mature Schwann cells, and explain why this unusual feature is central to the striking regenerative potential of the peripheral nervous system (PNS).
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Allodi I, Udina E, Navarro X. 2012. Specificity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol 98: 16–37. - PubMed
-
- Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ. 2007. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13: 2863–2870. - PubMed
-
- Atanasoski S, Shumas S, Dickson C, Scherer SS, Suter U. 2001. Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol Cell Neurosci 18: 581–592. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
