DAP12 Stabilizes the C-terminal Fragment of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) and Protects against LPS-induced Pro-inflammatory Response
- PMID: 25957402
- PMCID: PMC4505493
- DOI: 10.1074/jbc.M115.645986
DAP12 Stabilizes the C-terminal Fragment of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) and Protects against LPS-induced Pro-inflammatory Response
Abstract
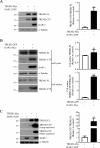
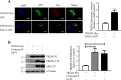
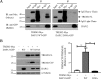
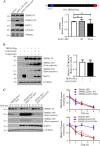
Triggering receptor expressed on myeloid cells 2 (TREM2) is a DAP12-associated receptor expressed in microglia, macrophages, and other myeloid-derived cells. Previous studies have suggested that TREM2/DAP12 signaling pathway reduces inflammatory responses and promotes phagocytosis of apoptotic neurons. Recently, TREM2 has been identified as a risk gene for Alzheimer disease (AD). Here, we show that DAP12 stabilizes the C-terminal fragment of TREM2 (TREM2-CTF), a substrate for γ-secretase. Co-expression of DAP12 with TREM2 selectively increased the level of TREM2-CTF with little effects on that of full-length TREM2. The interaction between DAP12 and TREM2 is essential for TREM2-CTF stabilization as a mutant form of DAP12 with disrupted interaction with TREM2 failed to exhibit such an effect. Silencing of either Trem2 or Dap12 gene significantly exacerbated pro-inflammatory responses induced by lipopolysaccharides (LPS). Importantly, overexpression of either full-length TREM2 or TREM2-CTF reduced LPS-induced inflammatory responses. Taken together, our results support a role of DAP12 in stabilizing TREM2-CTF, thereby protecting against excessive pro-inflammatory responses.
Keywords: Alzheimer disease; DAP12; TREM-CTF; TREM2; cytokine; inflammation; lipopolysaccharide (LPS); microglia; γ-secretase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Colonna M. (2003) TREMs in the immune system and beyond. Nat. Rev. Immunol. 3, 445–453 - PubMed
-
- Lanier L. L., Corliss B. C., Wu J., Leong C., Phillips J. H. (1998) Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 - PubMed
-
- Paloneva J., Autti T., Raininko R., Partanen J., Salonen O., Puranen M., Hakola P., Haltia M. (2001) CNS manifestations of Nasu-Hakola disease: a frontal dementia with bone cysts. Neurology 56, 1552–1558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

