CSN6 deregulation impairs genome integrity in a COP1-dependent pathway
- PMID: 25957415
- PMCID: PMC4494904
- DOI: 10.18632/oncotarget.3151
CSN6 deregulation impairs genome integrity in a COP1-dependent pathway
Abstract
Understanding genome integrity and DNA damage response are critical to cancer treatment. In this study, we identify CSN6's biological function in regulating genome integrity. Constitutive photomorphogenic 1 (COP1), an E3 ubiquitin ligase regulated by CSN6, is downregulated by DNA damage, but the biological consequences of this phenomenon are poorly understood. p27(Kip1) is a critical CDK inhibitor involved in cell cycle regulation, but its response to DNA damage remains unclear. Here, we report that p27(Kip1) levels are elevated after DNA damage, with concurrent reduction of COP1 levels. Mechanistic studies showed that during DNA damage response COP1's function as an E3 ligase of p27 is compromised, thereby reducing the ubiquitin-mediated degradation of p27(Kip1). Also, COP1 overexpression leads to downregulation of p27(Kip1), thereby promoting the expression of mitotic kinase Aurora A. Overexpression of Aurora A correlates with poor survival. These findings provide new insight into CSN6-COP1-p27(Kip1)-Aurora A axis in DNA damage repair and tumorigenesis.
Keywords: 14-3-3σ; COP1; DNA damage; p27; ubiquitination.
Figures
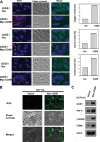
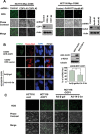
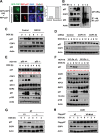
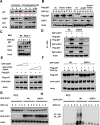
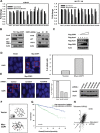
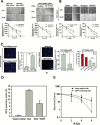
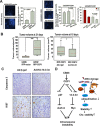
References
-
- Yoshida A, Kato JY, Nakamae I, Yoneda-Kato N. COP1 targets C/EBPalpha for degradation and induces acute myeloid leukemia via Trib1. Blood. 2013;122:1750–1760. - PubMed
-
- Jin G, Yamazaki Y, Takuwa M, Takahara T, Kaneko K, Kuwata T, Miyata S, Nakamura T. Trib1 and Evi1 cooperate with Hoxa and Meis1 in myeloid leukemogenesis. Blood. 2007;109:3998–4005. - PubMed
-
- Zhang XC, Chen J, Su CH, Yang HY, Lee MH. Roles for CSN5 in control of p53/MDM2 activities. J Cell Biochem. 2008;103:1219–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

