The Phosphate Binder Ferric Citrate and Mineral Metabolism and Inflammatory Markers in Maintenance Dialysis Patients: Results From Prespecified Analyses of a Randomized Clinical Trial
- PMID: 25958079
- PMCID: PMC4868355
- DOI: 10.1053/j.ajkd.2015.03.013
The Phosphate Binder Ferric Citrate and Mineral Metabolism and Inflammatory Markers in Maintenance Dialysis Patients: Results From Prespecified Analyses of a Randomized Clinical Trial
Abstract
Background: Phosphate binders are the cornerstone of hyperphosphatemia management in dialysis patients. Ferric citrate is an iron-based oral phosphate binder that effectively lowers serum phosphorus levels.
Study design: 52-week, open-label, phase 3, randomized, controlled trial for safety-profile assessment.
Setting & participants: Maintenance dialysis patients with serum phosphorus levels ≥6.0 mg/dL after washout of prior phosphate binders.
Intervention: 2:1 randomization to ferric citrate or active control (sevelamer carbonate and/or calcium acetate).
Outcomes: Changes in mineral bone disease, protein-energy wasting/inflammation, and occurrence of adverse events after 1 year.
Measurements: Serum calcium, intact parathyroid hormone, phosphorus, aluminum, white blood cell count, percentage of lymphocytes, serum urea nitrogen, and bicarbonate.
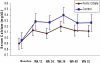
Results: There were 292 participants randomly assigned to ferric citrate, and 149, to active control. Groups were well matched. For mean changes from baseline, phosphorus levels decreased similarly in the ferric citrate and active control groups (-2.04±1.99 [SD] vs -2.18±2.25 mg/dL, respectively; P=0.9); serum calcium levels increased similarly in the ferric citrate and active control groups (0.22±0.90 vs 0.31±0.95 mg/dL; P=0.2). Hypercalcemia occurred in 4 participants receiving calcium acetate. Parathyroid hormone levels decreased similarly in the ferric citrate and active control groups (-167.1±399.8 vs -152.7±392.1 pg/mL; P=0.8). Serum albumin, bicarbonate, serum urea nitrogen, white blood cell count and percentage of lymphocytes, and aluminum values were similar between ferric citrate and active control. Total and low-density lipoprotein cholesterol levels were lower in participants receiving sevelamer than those receiving ferric citrate and calcium acetate. Fewer participants randomly assigned to ferric citrate had serious adverse events compared with active control.
Limitations: Open-label study, few peritoneal dialysis patients.
Conclusions: Ferric citrate was associated with similar phosphorus control compared to active control, with similar effects on markers of bone and mineral metabolism in dialysis patients. There was no evidence of protein-energy wasting/inflammation or aluminum toxicity, and fewer participants randomly assigned to ferric citrate had serious adverse events. Ferric citrate is an effective phosphate binder with a safety profile comparable to sevelamer and calcium acetate.
Keywords: Hemodialysis; adverse events; calcium acetate; end-stage renal disease (ESRD); ferric citrate; hyperphosphatemia; mineral bone disease; phosphate binder; protein-energy wasting (PEW)/inflammation; safety; sevelamer carbonate.
Copyright © 2015 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. - PubMed
-
- National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4)(suppl 3):S1–S201. - PubMed
-
- Boyce B, Fell G, Elder H, et al. Hypercalcaemic osteomalacia due to aluminum toxicity. Lancet. 1982;2:1009–1013. - PubMed
-
- Swartz R, Dombrouski J, Burnotowska M, Mayor G. Microcytic anemia in dialysis patients: reversible marker of aluminum toxicity. Am J Kidney Dis. 1987;9:217–223. - PubMed
-
- Alfrey A, LeGendre G, Kaehny W. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N Engl J Med. 1976;294:184–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

