Botulinum toxin in migraine: Role of transport in trigemino-somatic and trigemino-vascular afferents
- PMID: 25958249
- PMCID: PMC4458441
- DOI: 10.1016/j.nbd.2015.04.011
Botulinum toxin in migraine: Role of transport in trigemino-somatic and trigemino-vascular afferents
Abstract
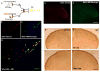
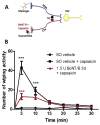
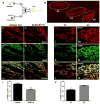
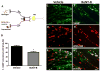
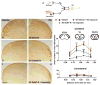
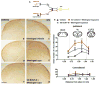
Migraine secondary to meningeal input is referred to extracranial regions innervated by somatic afferents that project to homologous regions in the trigeminal nucleus caudalis (TNC). Reported efficacy of extracranial botulinum toxin (BoNT) in treating migraine is surprising since a local extracranial effect of BoNT cannot account for its effect upon meningeal input. We hypothesize that intradermal BoNT acts through central transport in somatic afferents. Anesthetized C57Bl/6 mice (male) received unilateral supraorbital (SO) injections of BoNT-B (1.5 U/40 μl) or saline. 3 days later, mice received ipsilateral (ipsi)-SO capsaicin (20 μl of 0.5mM solution) or meningeal capsaicin (4 μl of 0.35 μM). Pre-treatment with ipsi-SO BoNT-B i) decreased nocicsponsive ipsilateral wiping behavior following ipsi-SO capsaicin; ii) produced cleavage of VAMP in the V1 region of ipsi-TG and in TG neurons showing WGA after SO injection; iii) reduced expression of c-fos in ipsi-TNC following ipsi-SO capsaicin; iv) reduced c-fos activation and NK-1 internalization in ipsi-TNC secondary to ipsi-meningeal capsaicin; and vi) SO WGA did not label dural afferents. We conclude that BoNT-B is taken up by peripheral afferents and transported to central terminals where it inhibits transmitter release resulting in decreased activation of second order neurons. Further, this study supports the hypothesis that SO BoNT exerts a trans-synaptic action on either the second order neuron (which receives convergent input from the meningeal afferent) or the terminal/TG of the converging meningeal afferent.
Keywords: Botulinum toxin; Migraine; Referred pain; Trans-synaptic; Transcytosis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
We declare that there is no conflict of financial interest with regard to or manuscript.
Figures



Similar articles
-
Therapeutic use of botulinum toxin in migraine: mechanisms of action.Br J Pharmacol. 2014 Sep;171(18):4177-92. doi: 10.1111/bph.12763. Br J Pharmacol. 2014. PMID: 24819339 Free PMC article. Review.
-
Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons.Pain. 2014 Aug;155(8):1516-1526. doi: 10.1016/j.pain.2014.04.027. Epub 2014 May 2. Pain. 2014. PMID: 24793910
-
Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors' responses to stimulation of TRPV1 and TRPA1 channels: Are we getting closer to solving this puzzle?Cephalalgia. 2016 Aug;36(9):875-86. doi: 10.1177/0333102416636843. Epub 2016 Mar 16. Cephalalgia. 2016. PMID: 26984967 Free PMC article.
-
Botulinum toxin B in the sensory afferent: transmitter release, spinal activation, and pain behavior.Pain. 2014 Apr;155(4):674-684. doi: 10.1016/j.pain.2013.12.009. Epub 2013 Dec 11. Pain. 2014. PMID: 24333775 Free PMC article.
-
Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine.Brain Res Brain Res Rev. 2001 Mar;35(1):20-35. doi: 10.1016/s0165-0173(00)00048-5. Brain Res Brain Res Rev. 2001. PMID: 11245884 Review.
Cited by
-
A Study and Review of Effects of Botulinum Toxins on Mast Cell Dependent and Independent Pruritus.Toxins (Basel). 2018 Mar 23;10(4):134. doi: 10.3390/toxins10040134. Toxins (Basel). 2018. PMID: 29570628 Free PMC article.
-
Mechanisms of Botulinum Toxin Type A Action on Pain.Toxins (Basel). 2019 Aug 5;11(8):459. doi: 10.3390/toxins11080459. Toxins (Basel). 2019. PMID: 31387301 Free PMC article. Review.
-
The Expanding Therapeutic Utility of Botulinum Neurotoxins.Toxins (Basel). 2018 May 18;10(5):208. doi: 10.3390/toxins10050208. Toxins (Basel). 2018. PMID: 29783676 Free PMC article. Review.
-
Effects of intraplantar botulinum toxin-B on carrageenan-induced changes in nociception and spinal phosphorylation of GluA1 and Akt.Eur J Neurosci. 2016 Jul;44(1):1714-22. doi: 10.1111/ejn.13261. Epub 2016 May 19. Eur J Neurosci. 2016. PMID: 27108664 Free PMC article.
-
Botulinum Toxin in the Treatment of Headache.Toxins (Basel). 2020 Dec 17;12(12):803. doi: 10.3390/toxins12120803. Toxins (Basel). 2020. PMID: 33348571 Free PMC article. Review.
References
-
- Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl 1):S9–15. - PubMed
-
- Bach-Rojecky L, Lackovic Z. Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J. 2005;46(2):201–208. - PubMed
-
- Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94(2):234–238. - PubMed
-
- Bach-Rojecky L, Salkovic-Petrisic M, Lackovic Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: bilateral effect after unilateral injection. Eur J Pharmacol. 2010;633(1–3):10–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

