Evolution of two prototypic T cell lineages
- PMID: 25958271
- PMCID: PMC4466127
- DOI: 10.1016/j.cellimm.2015.04.007
Evolution of two prototypic T cell lineages
Abstract
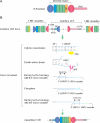
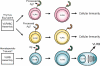
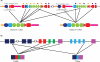
Jawless vertebrates, which occupy a unique position in chordate phylogeny, employ leucine-rich repeat (LRR)-based variable lymphocyte receptors (VLR) for antigen recognition. During the assembly of the VLR genes (VLRA, VLRB and VLRC), donor LRR-encoding sequences are copied in a step-wise manner into the incomplete germ-line genes. The assembled VLR genes are differentially expressed by discrete lymphocyte lineages: VLRA- and VLRC-producing cells are T-cell like, whereas VLRB-producing cells are B-cell like. VLRA(+) and VLRC(+) lymphocytes resemble the two principal T-cell lineages of jawed vertebrates that express the αβ or γδ T-cell receptors (TCR). Reminiscent of the interspersed nature of the TCRα/TCRδ locus in jawed vertebrates, the close proximity of the VLRA and VLRC loci facilitates sharing of donor LRR sequences during VLRA and VLRC assembly. Here we discuss the insight these findings provide into vertebrate T- and B-cell evolution, and the alternative types of anticipatory receptors they use for adaptive immunity.
Keywords: Adaptive immunity; Jawless vertebrates; Leucine-rich repeat; Variable lymphocyte receptor; αβ and γδ T-cell receptor.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

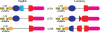

References
-
- Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. - PubMed
-
- Boffa GA, Fine JM, Drilhon A, Amouch P. Immunoglobulins and transferrin in marine lamprey sera. Nature. 1967;214:700–702. - PubMed
-
- Pollara B, Litman GW, Finstad J, Howell J, Good RA. The evolution of the immune response. VII. Antibody to human “O” cells and properties of the immunoglobulin in lamprey. J. Immunol. 1970;105:738–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

