A phase I study of IV doxorubicin plus intraperitoneal (IP) paclitaxel and IV or IP cisplatin in endometrial cancer patients at high risk for peritoneal failure (GOG 9920): an NRG Oncology/Gynecologic Oncology Group study
- PMID: 25958319
- PMCID: PMC4643744
- DOI: 10.1016/j.ygyno.2015.04.038
A phase I study of IV doxorubicin plus intraperitoneal (IP) paclitaxel and IV or IP cisplatin in endometrial cancer patients at high risk for peritoneal failure (GOG 9920): an NRG Oncology/Gynecologic Oncology Group study
Abstract
Objective: To determine the maximum tolerated dose (MTD) of a modified paclitaxel/doxorubicin/cisplatin (TAP) regimen which incorporated intraperitoneal (IP) paclitaxel or IP paclitaxel/cisplatin in advanced endometrial cancer.
Methods: Patients (pts) with FIGO (1998) Stage IIIA/IIIC with positive cytologic washings/ascites, adnexa, or serosa or Stage IV (intraperitoneal disease spread), histologically confirmed endometrial cancer were eligible. The study was designed as a phase I, 3+3 dose escalation study evaluating 5 dose levels (DL). All pts received cycles 1-2 with IV TAP, and cycles 3-6 with IV/IP therapy, on a 21day schedule. Adverse events were evaluated on cycles 3-4 for dose limiting toxicity (DLT) and dose escalation decisions.
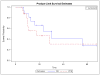
Results: Twenty-one pts were enrolled, of which 17 were evaluable for DLT. Most pts had Stage IV disease (76%) and serous/clear cell histology (59%). The MTD was determined to be DL 3 (cycles 3-6 including paclitaxel 90mg/m(2) IP, doxorubicin 45mg/m(2) IV, cisplatin 50mg/m(2)). Three DLT events occurred and were related to grades 3-4 metabolic toxicities. There was one grade 2 sensory neuropathy event and myelosupression was tolerable without the use of G-CSF. 88% of evaluable pts completed 6cycles of therapy. With a median follow-up of 22months, 46% of patients remain progression-free at 2years.
Conclusion: We described an IV/IP based modification of a standard TAP regimen in endometrial cancer. Based on the high rate of completing 6cycles of therapy, low rates of neuropathy, and promising PFS, further study of IP therapy in endometrial cancer is warranted.
Keywords: Cisplatin; Doxorubicin plus intraperitoneal; Endometrial cancer patients; NRG Oncology.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors wish to report that there are no relevant conflicts of interests with the exception of Dr. Kathleen Moore who would like to disclose that she has received personal fees for being on the advisory boards of Astra Zeneca, Genentech/Roche, ImmunoGen, Advaxis and Amgen. Additionally, Dr. Premal Thaker reports that he has received personal fees from Incyte as a member of the Data Advisory Board as well as Celsion for his role as a Consultant.
Figures
Similar articles
-
Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer.Gynecol Oncol. 2012 Jun;125(3):621-4. doi: 10.1016/j.ygyno.2012.03.027. Epub 2012 Mar 21. Gynecol Oncol. 2012. PMID: 22446622
-
Phase I trial of the treatment of high-risk endometrial cancer with concurrent weekly paclitaxel and cisplatin and whole abdominal radiation therapy: a Gynecologic Oncology Group study.Gynecol Oncol. 2009 Jan;112(1):134-41. doi: 10.1016/j.ygyno.2008.09.015. Epub 2008 Oct 29. Gynecol Oncol. 2009. PMID: 18962846 Clinical Trial.
-
Feasibility study of combination chemotherapy with paclitaxel, doxorubicin and cisplatin without prophylactic granulocyte colony-stimulating factor injection for intermediate-to-high risk or recurrent endometrial cancer.Jpn J Clin Oncol. 2014 Nov;44(11):1040-4. doi: 10.1093/jjco/hyu124. Epub 2014 Sep 2. Jpn J Clin Oncol. 2014. PMID: 25183770 Clinical Trial.
-
The platinum compounds and paclitaxel in the management of carcinomas of the endometrium and uterine cervix.Semin Oncol. 1995 Oct;22(5 Suppl 12):67-75. Semin Oncol. 1995. PMID: 7481864 Review.
-
New aspects of adjuvant therapy in endometrial cancer: current standards and future directions.Crit Rev Oncol Hematol. 2008 Sep;67(3):204-12. doi: 10.1016/j.critrevonc.2008.02.011. Epub 2008 Apr 14. Crit Rev Oncol Hematol. 2008. PMID: 18407513 Review.
Cited by
-
Recurrent Endometrial Cancer: Local and Systemic Treatment Options.Cancers (Basel). 2021 Dec 14;13(24):6275. doi: 10.3390/cancers13246275. Cancers (Basel). 2021. PMID: 34944893 Free PMC article. Review.
-
The addition of paclitaxel to doxorubicin and cisplatin and volume-directed radiation does not improve overall survival (OS) or long-term recurrence-free survival (RFS) in advanced endometrial cancer (EC): A randomized phase III NRG/Gynecologic Oncology Group (GOG) study.Gynecol Oncol. 2019 Jul;154(1):13-21. doi: 10.1016/j.ygyno.2019.03.240. Epub 2019 Apr 30. Gynecol Oncol. 2019. PMID: 31053405 Free PMC article. Clinical Trial.
-
Clinical trials in gynecologic oncology: Past, present, and future.Gynecol Oncol. 2018 Feb;148(2):393-402. doi: 10.1016/j.ygyno.2017.11.026. Epub 2017 Dec 6. Gynecol Oncol. 2018. PMID: 29212614 Free PMC article. Review.
References
-
- Creasman WT, Morrow CP, Bundy B, et al. Surgical Pathological Spread Patterns of Endometrial Cancer: A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–41. - PubMed
-
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. Springer; New York: 2010. Corpus uteri; pp. 403–409.
-
- Nelson G, Randall M, Sutton G, et al. FIGO Stage IIIC endometrial cancer with metastases confined to pelvic lymph nodes: analysis of treatment outcomes, prognostic variables, and failure patterns following adjuvant radiation therapy. Gynecol Oncol. 1999;75:211–217. - PubMed
-
- McMeekin DS, Lashbrook D, Gold M, et al. Analysis of FIGO Stage IIIC endometrial cancer patients. Gynecol Oncol. 2001;81:273–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous