NBPF1, a tumor suppressor candidate in neuroblastoma, exerts growth inhibitory effects by inducing a G1 cell cycle arrest
- PMID: 25958384
- PMCID: PMC4440459
- DOI: 10.1186/s12885-015-1408-5
NBPF1, a tumor suppressor candidate in neuroblastoma, exerts growth inhibitory effects by inducing a G1 cell cycle arrest
Abstract
Background: NBPF1 (Neuroblastoma Breakpoint Family, member 1) was originally identified in a neuroblastoma patient on the basis of its disruption by a chromosomal translocation t(1;17)(p36.2;q11.2). Considering this genetic defect and the frequent genomic alterations of the NBPF1 locus in several cancer types, we hypothesized that NBPF1 is a tumor suppressor. Decreased expression of NBPF1 in neuroblastoma cell lines with loss of 1p36 heterozygosity and the marked decrease of anchorage-independent clonal growth of DLD1 colorectal carcinoma cells with induced NBPF1 expression further suggest that NBPF1 functions as tumor suppressor. However, little is known about the mechanisms involved.
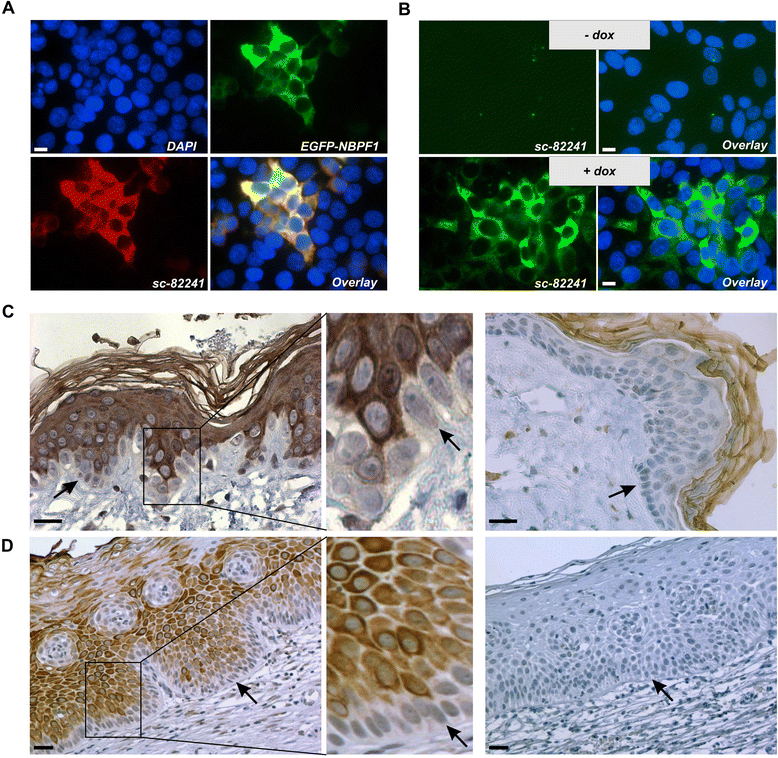
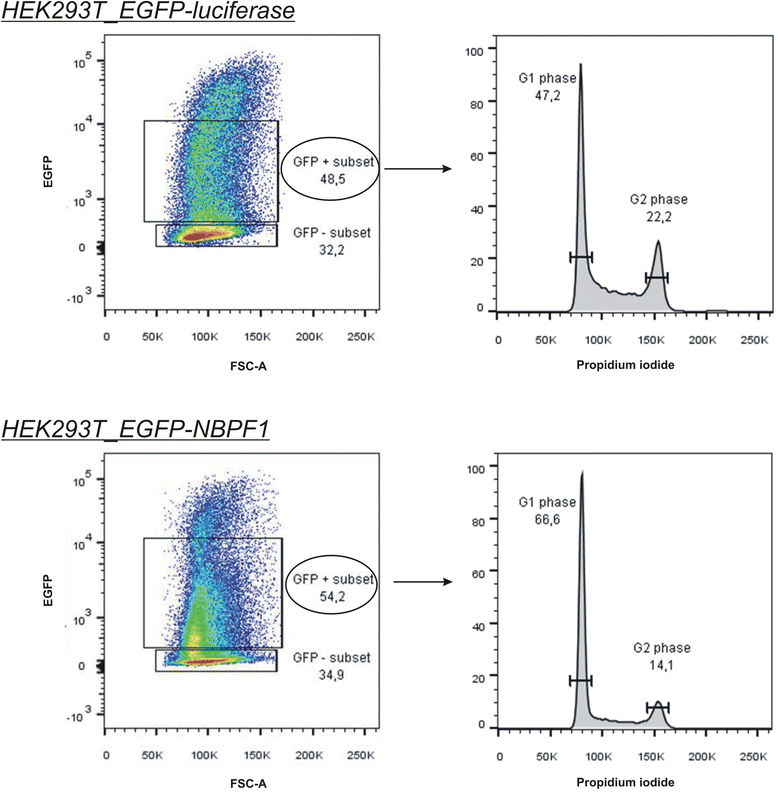
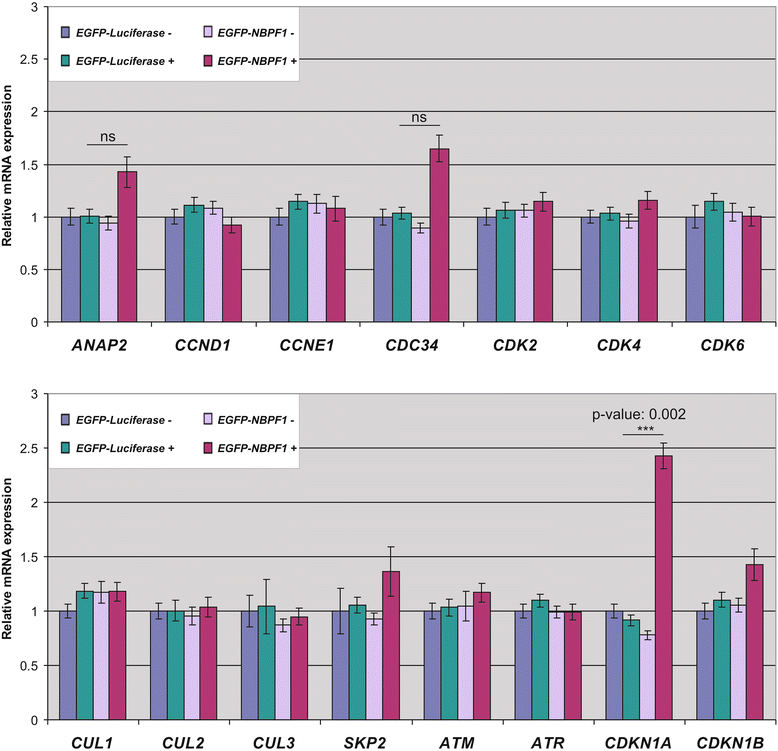
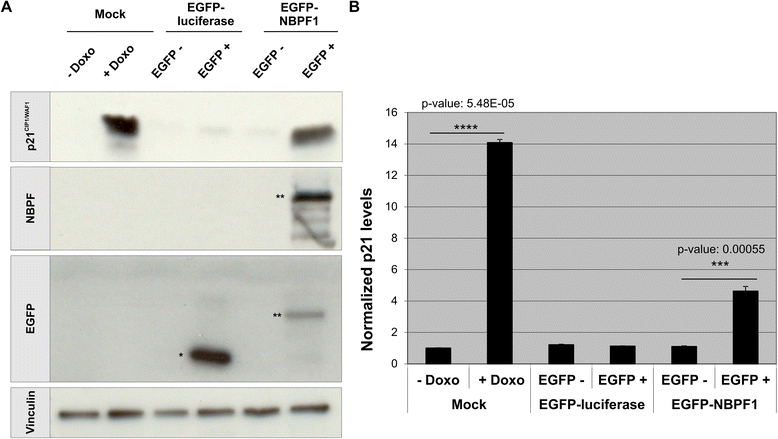
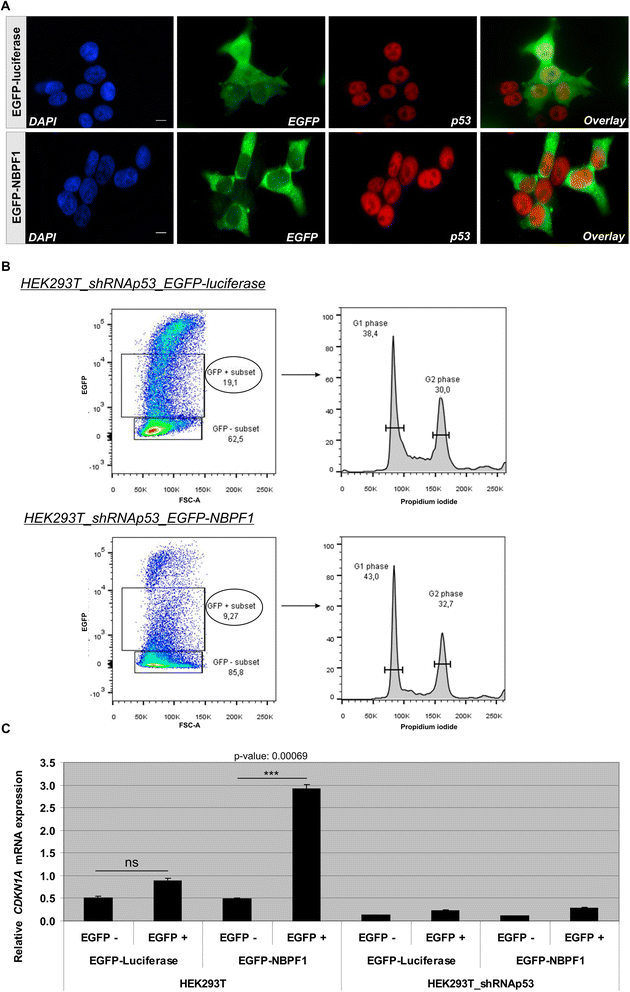
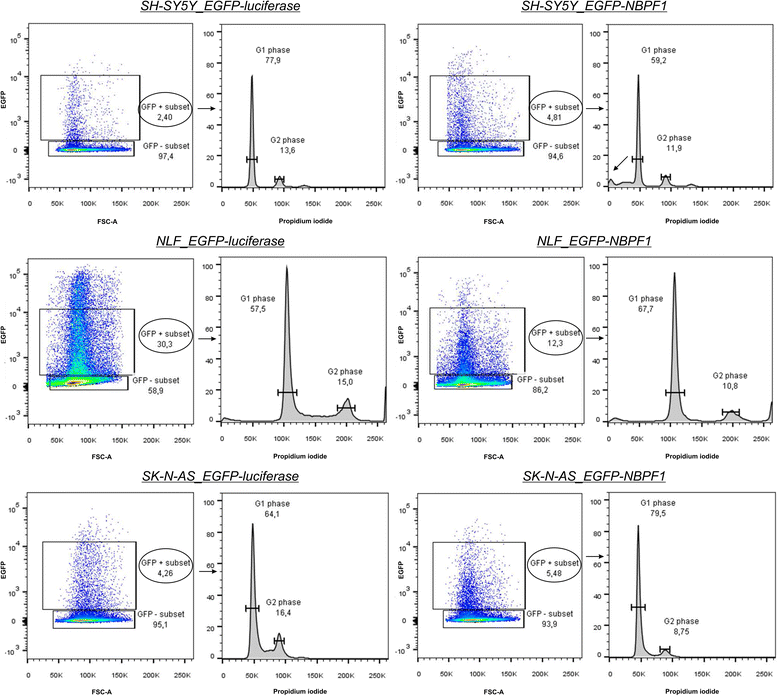
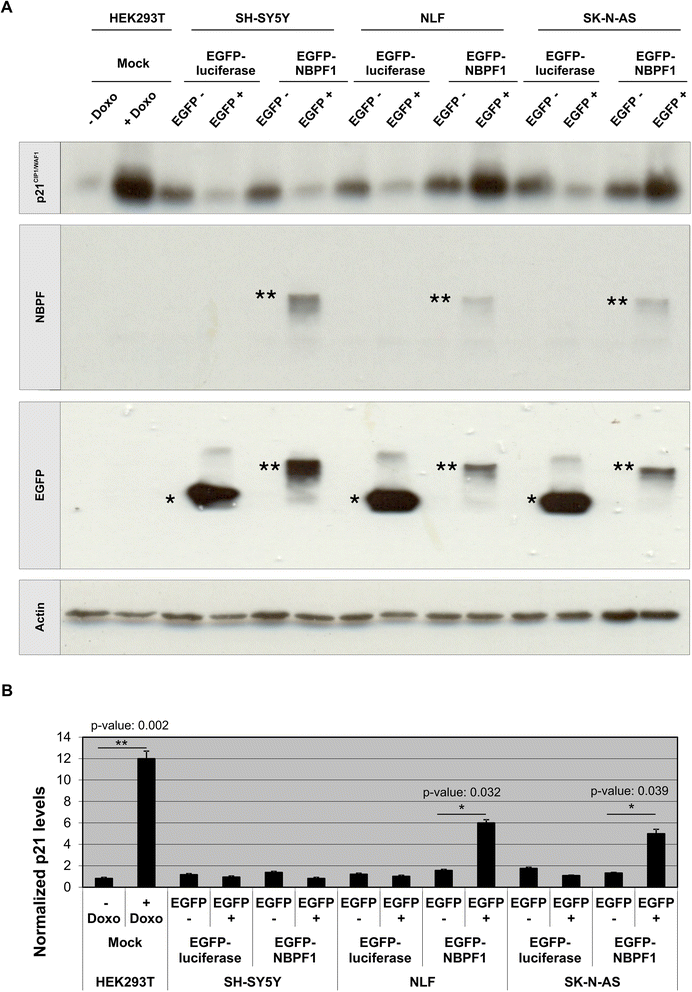
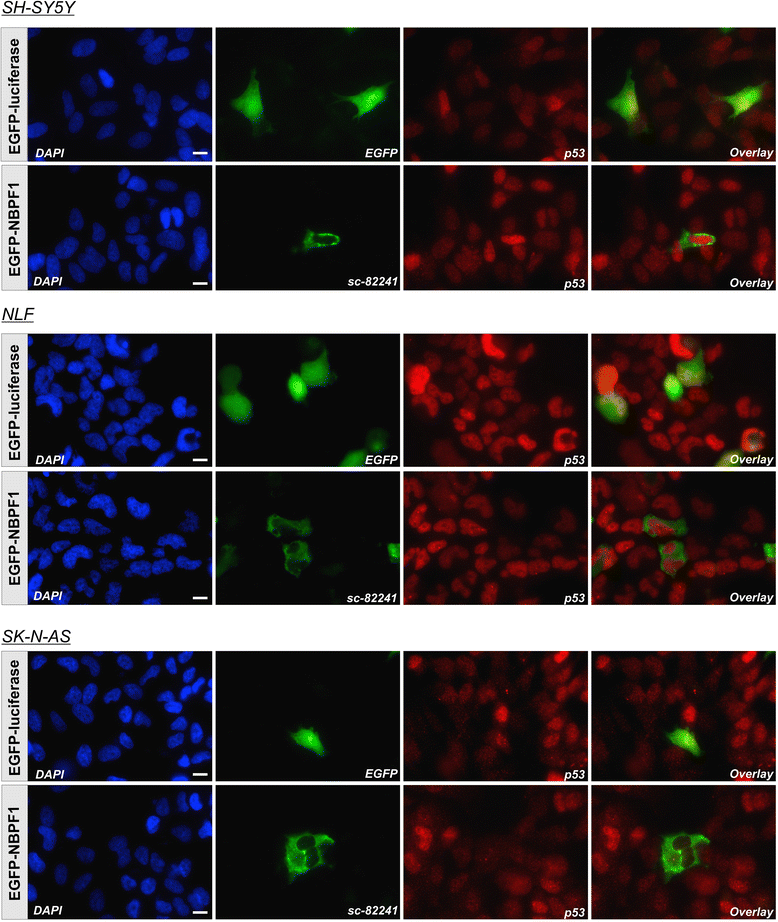
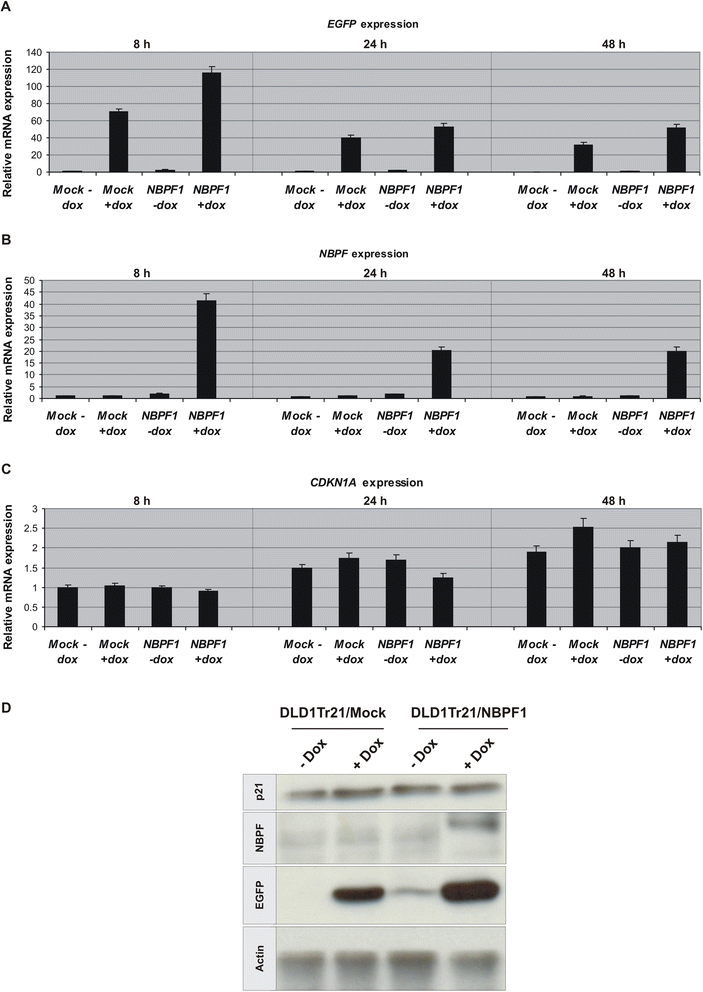
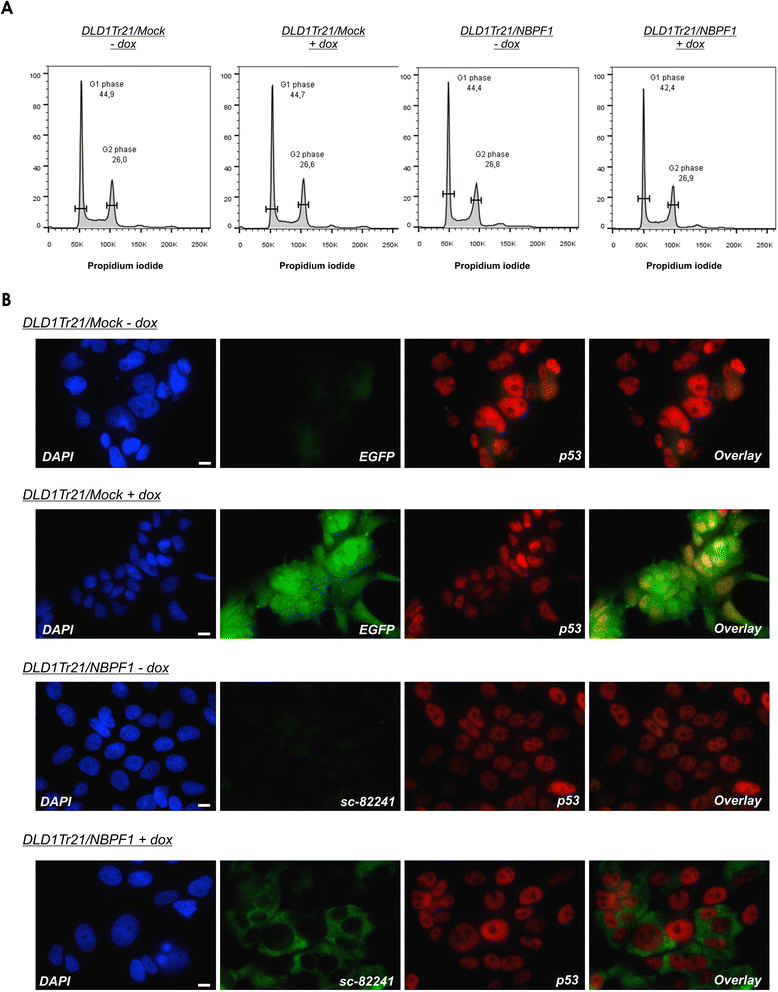
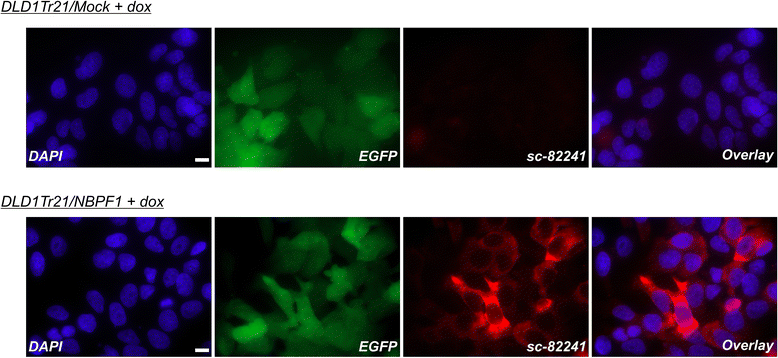
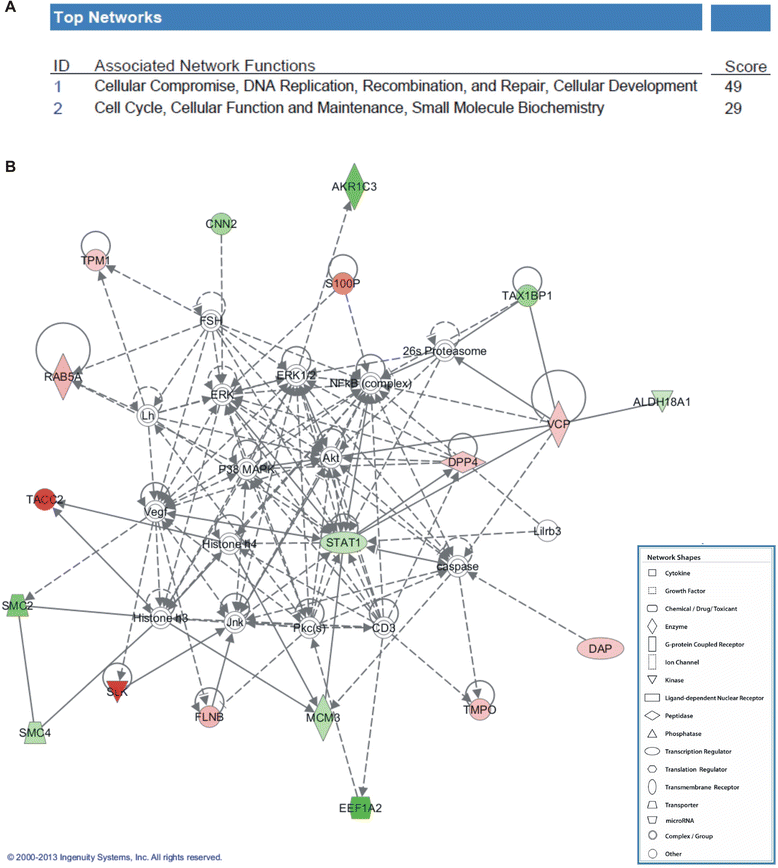
Methods: Expression of NBPF was analyzed in human skin and human cervix by immunohistochemistry. The effects of NBPF1 on the cell cycle were evaluated by flow cytometry. We investigated by real-time quantitative RT-PCR the expression profile of a panel of genes important in cell cycle regulation. Protein levels of CDKN1A-encoded p21(CIP1/WAF1) were determined by western blotting and the importance of p53 was shown by immunofluorescence and by a loss-of-function approach. LC-MS/MS analysis was used to investigate the proteome of DLD1 colon cancer cells with induced NBPF1 expression. Possible biological interactions between the differentially regulated proteins were investigated with the Ingenuity Pathway Analysis tool.
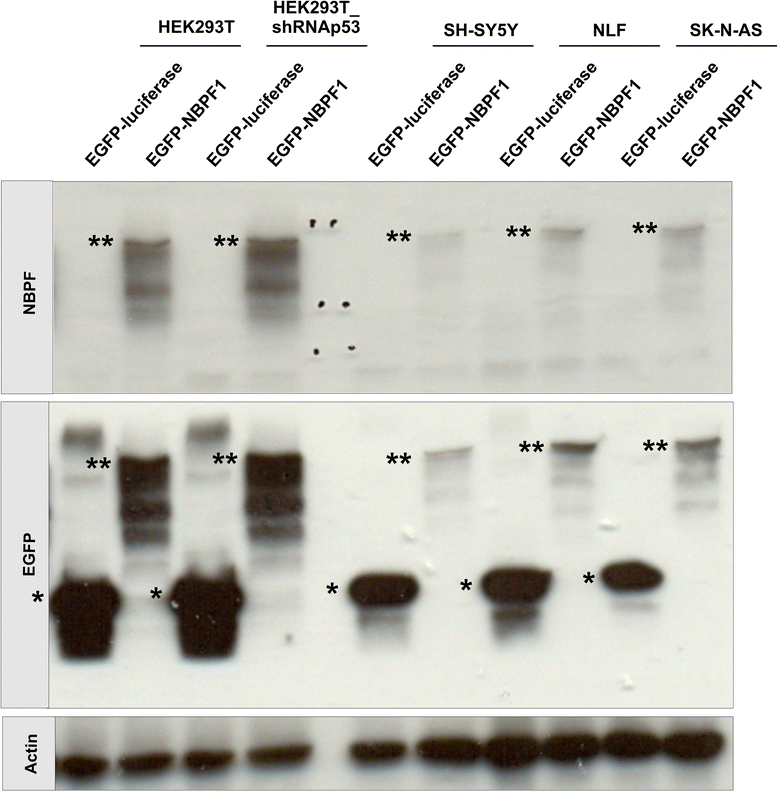
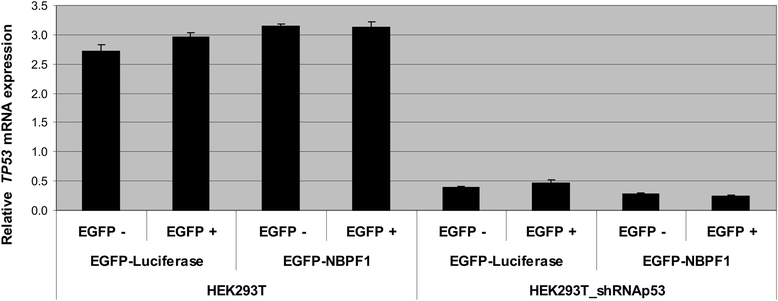
Results: We show that NBPF is expressed in the non-proliferative suprabasal layers of squamous stratified epithelia of human skin and cervix. Forced expression of NBPF1 in HEK293T cells resulted in a G1 cell cycle arrest that was accompanied by upregulation of the cyclin-dependent kinase inhibitor p21(CIP1/WAF1) in a p53-dependent manner. Additionally, forced expression of NBPF1 in two p53-mutant neuroblastoma cell lines also resulted in a G1 cell cycle arrest and CDKN1A upregulation. However, CDKN1A upregulation by NBPF1 was not observed in the DLD1 cells, which demonstrates that NBPF1 exerts cell-specific effects. In addition, proteome analysis of NBPF1-overexpressing DLD1 cells identified 32 differentially expressed proteins, of which several are implicated in carcinogenesis.
Conclusions: We demonstrated that NBPF1 exerts different tumor suppressive effects, depending on the cell line analyzed, and provide new clues into the molecular mechanism of the enigmatic NBPF proteins.
Figures
References
-
- Laureys G, Speleman F, Versteeg R, van der Drift P, Chan A, Leroy J, et al. Constitutional translocation t(1;17)(p36.31-p36.13;q11.2-q12.1) in a neuroblastoma patient. Establishment of somatic cell hybrids and identification of PND/A12M2 on chromosome 1 and NF1/SCYA7 on chromosome 17 as breakpoint flanking single copy markers. Oncogene. 1995;10:1087–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

