Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation
- PMID: 25959047
- PMCID: PMC4430126
- DOI: 10.1038/ncomms7931
Macrophages monitor tissue osmolarity and induce inflammatory response through NLRP3 and NLRC4 inflammasome activation
Abstract
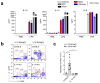
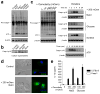
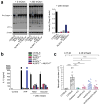
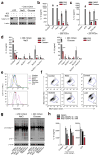
Interstitial osmolality is a key homeostatic variable that varies depending on the tissue microenvironment. Mammalian cells have effective mechanisms to cope with osmotic stress by engaging various adaptation responses. Hyperosmolality due to high dietary salt intake has been linked to pathological inflammatory conditions. Little is known about the mechanisms of sensing the hyperosmotic stress by the innate immune system. Here we report that caspase-1 is activated in macrophages under hypertonic conditions. Mice with high dietary salt intake display enhanced induction of Th17 response upon immunization, and this effect is abolished in caspase-1-deficient mice. Our findings identify an unknown function of the inflammasome as a sensor of hyperosmotic stress, which is crucial for the induction of inflammatory Th17 response.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

