Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1
- PMID: 25959226
- PMCID: PMC4449299
- DOI: 10.1016/j.devcel.2015.03.003
Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1
Abstract
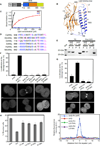
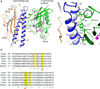
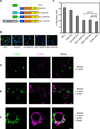
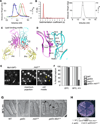
Anillins and Mid1 are scaffold proteins that play key roles in anchorage of the contractile ring at the cell equator during cytokinesis in animals and fungi, respectively. Here, we report crystal structures and functional analysis of human anillin and S. pombe Mid1. The combined data show anillin contains a cryptic C2 domain and a Rho-binding domain. Together with the tethering PH domain, three membrane-associating elements synergistically bind to RhoA and phospholipids to anchor anillin at the cleavage furrow. Surprisingly, Mid1 also binds to the membrane through a cryptic C2 domain. Dimerization of Mid1 leads to high affinity and preference for PI(4,5)P2, which stably anchors Mid1 at the division plane, bypassing the requirement for Rho GTPase. These findings uncover the unexpected general machinery and the divergent regulatory logics for the anchorage of the contractile ring through the anillin/Mid1 family proteins from yeast to humans.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

