Mechanism of Transcription Termination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element
- PMID: 25959395
- PMCID: PMC4475470
- DOI: 10.1016/j.molcel.2015.04.002
Mechanism of Transcription Termination by RNA Polymerase III Utilizes a Non-template Strand Sequence-Specific Signal Element
Abstract
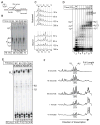
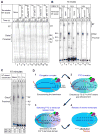
Understanding the mechanism of transcription termination by a eukaryotic RNA polymerase (RNAP) has been limited by lack of a characterizable intermediate that reflects transition from an elongation complex to a true termination event. While other multisubunit RNAPs require multipartite cis-signals and/or ancillary factors to mediate pausing and release of the nascent transcript from the clutches of these enzymes, RNAP III does so with precision and efficiency on a simple oligo(dT) tract, independent of other cis-elements or trans-factors. We report an RNAP III pre-termination complex that reveals termination mechanisms controlled by sequence-specific elements in the non-template strand. Furthermore, the TFIIF-like RNAP III subunit C37 is required for this function of the non-template strand signal. The results reveal the RNAP III terminator as an information-rich control element. While the template strand promotes destabilization via a weak oligo(rU:dA) hybrid, the non-template strand provides distinct sequence-specific destabilizing information through interactions with the C37 subunit.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Creative Math of RNA Polymerase III Termination: Sense Plus Antisense Makes More Sense.Mol Cell. 2015 Jun 18;58(6):974-6. doi: 10.1016/j.molcel.2015.06.003. Mol Cell. 2015. PMID: 26091347
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

