Pharmacokinetics of tenofovir during pregnancy and postpartum
- PMID: 25959631
- PMCID: PMC4862736
- DOI: 10.1111/hiv.12252
Pharmacokinetics of tenofovir during pregnancy and postpartum
Abstract
Objectives: Tenofovir disoproxil fumarate (TDF) is increasingly used in the highly active antiretroviral therapy (HAART) regimens of pregnant women, but limited data exist on the pregnancy pharmacokinetics of chronically dosed TDF. This study described tenofovir pharmacokinetics during pregnancy and postpartum.
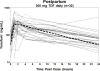
Methods: International Maternal Pediatric and Adolescent AIDS Clinical Trials (IMPAACT) P1026s is a prospective, nonblinded pharmacokinetic study of HIV-infected pregnant women that included a cohort receiving 300 mg TDF once daily. Steady-state 24-hour pharmacokinetic profiles were measured at the second and third trimesters, postpartum, and in maternal and umbilical cord samples collected at delivery. Tenofovir was measured by liquid chromatography-mass spectrometry (LC-MS). The target area under the concentration versus time curve from time 0 to 24 h post dose (AUC) was ≥ 1.99 μg h/mL (nonpregnant historical control 10th percentile).
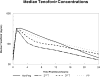
Results: The median tenofovir AUC was decreased during the second (1.9 μg h/mL) and third (2.4 μg h/mL; P = 0.005) trimesters versus postpartum (3.0 μg h/mL). Tenofovir AUC exceeded the target for two of four women (50%) in the second trimester, 27 of 37 women [73%; 95% confidence interval (CI) 56%, 86%] in the third trimester, and 27 of 32 women (84%; 95% CI 67%, 95%) postpartum (P > 0.05). Median second/third-trimester troughs were lower (39/54 ng/mL) than postpartum (61 ng/mL). Median third-trimester weight was greater for subjects below the target AUC versus those above the target (97.9 versus 74.2 kg, respectively; P = 0.006). The median ratio of cord blood to maternal concentrations was 0.88. No infants were HIV infected.
Conclusions: This study found lower tenofovir AUC and troughs during pregnancy. Transplacental passage with chronic TDF use during pregnancy was high. Standard TDF doses appear to be appropriate for most HIV-infected pregnant women but therapeutic drug monitoring with dose adjustment should be considered in pregnant women with high weight (> 90 kg) or inadequate HIV RNA response.
Keywords: HIV; antiretrovirals; pregnancy; prevention of perinatal transmission; tenofovir.
© 2015 British HIV Association.
Conflict of interest statement
Figures

References
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed July 9, 2013.
-
- Buckoreelall K, Cressey TR, King JR. Pharmacokinetic optimization of antiretroviral therapy in pregnancy. Clin Pharmacokinet. 2012;51:639–59. - PubMed
-
- Delahunty T, Bushman L, Fletcher CV. Sensitive assay for determining plasma tenofovir concentrations by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830:6–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR000124/TR/NCATS NIH HHS/United States
- UL1TR000423/TR/NCATS NIH HHS/United States
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- N01-DK-9-001/HHSN267200800001C/DK/NIDDK NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- AI068632/AI/NIAID NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- 1 U01 AI068616/AI/NIAID NIH HHS/United States
- 5 U01 AI41110/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

