Genetic dissection of a regionally differentiated network for exploratory behavior in Drosophila larvae
- PMID: 25959962
- PMCID: PMC4446794
- DOI: 10.1016/j.cub.2015.03.023
Genetic dissection of a regionally differentiated network for exploratory behavior in Drosophila larvae
Abstract
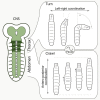
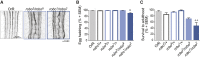
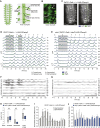
An efficient strategy to explore the environment for available resources involves the execution of random walks where straight line locomotion alternates with changes of direction. This strategy is highly conserved in the animal kingdom, from zooplankton to human hunter-gatherers. Drosophila larvae execute a routine of this kind, performing straight line crawling interrupted at intervals by pause turns that halt crawling and redirect the trajectory of movement. The execution of this routine depends solely on the activity of networks located in the thoracic and abdominal segments of the nervous system, while descending input from the brain serves to modify it in a context-dependent fashion. I used a genetic method to investigate the location and function of the circuitry required for the different elements of exploratory crawling. By using the Slit-Robo axon guidance pathway to target neuronal midline crossing defects selectively to particular regions of the thoracic and abdominal networks, it has been possible to define at least three functions required for the performance of the exploratory routine: (1) symmetrical outputs in thoracic and abdominal segments that generate the crawls; (2) asymmetrical output that is uniquely initiated in the thoracic segments and generates the turns; and (3) an intermittent interruption to crawling that determines the time-dependent transition between crawls and turns.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Garcia R., Moss F., Nihongi A., Strickler J.R., Göller S., Erdmann U., Schimansky-Geier L., Sokolov I.M. Optimal foraging by zooplankton within patches: the case of Daphnia. Math. Biosci. 2007;207:165–188. - PubMed
-
- McClellan A.D., Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J. Neurophysiol. 1997;78:214–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

