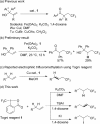
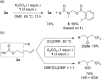
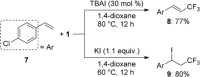
Product Control in Alkene Trifluoromethylation: Hydrotrifluoromethylation, Vinylic Trifluoromethylation, and Iodotrifluoromethylation using Togni Reagent
- PMID: 25960034
- PMCID: PMC4600224
- DOI: 10.1002/asia.201500359
Product Control in Alkene Trifluoromethylation: Hydrotrifluoromethylation, Vinylic Trifluoromethylation, and Iodotrifluoromethylation using Togni Reagent
Abstract
Hydrotrifluoromethylation, vinylic trifluoromethylation, and iodotrifluoromethylation of simple alkenes have been achieved by using Togni reagent in the absence of any transition metal catalyst. These reactions were readily controllable by selection of appropriate salts and solvents. The addition of K2CO3 afforded the hydrotrifluoromethylation product, with DMF acting not only as a solvent, but also as the hydrogen source. In contrast, the use of tetra-n-butylammonium iodide (TBAI) in 1,4-dioxane resulted in vinylic trifluoromethylation, while the use of KI afforded the iodotrifluoromethylation product. The vinylic trifluoromethylation product was obtained by treatment of the iodotrifluoromethylation product with ammonium 2-iodobenzoate, indicating that it was formed through an elimination reaction of the in-situ-generated iodotrifluoromethylation product, and the solubility of the resulting 2-iodobenzoate salt plays a key role in the product switching. A radical-clock experiment showed that these reactions proceed via radical intermediates.
Keywords: Togni reagent; additive effect; alkenes; difunctionalization; trifluoromethylation.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures
Similar articles
-
Hydrotrifluoromethylation and iodotrifluoromethylation of alkenes and alkynes using an inorganic electride as a radical generator.Nat Commun. 2014 Sep 12;5:4881. doi: 10.1038/ncomms5881. Nat Commun. 2014. PMID: 25216119
-
Copper-Catalyzed Trifluoromethylation of Alkoxypyridine Derivatives.Molecules. 2020 Oct 16;25(20):4766. doi: 10.3390/molecules25204766. Molecules. 2020. PMID: 33081411 Free PMC article.
-
Copper-mediated radical 1,2-bis(trifluoromethylation) of alkenes with sodium trifluoromethanesulfinate.Org Lett. 2015 Apr 17;17(8):1906-9. doi: 10.1021/acs.orglett.5b00601. Epub 2015 Mar 25. Org Lett. 2015. PMID: 25806665
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
A "Renaissance" in radical trifluoromethylation.Angew Chem Int Ed Engl. 2012 Sep 3;51(36):8950-8. doi: 10.1002/anie.201202624. Epub 2012 Aug 13. Angew Chem Int Ed Engl. 2012. PMID: 22890985 Review.
Cited by
-
Photocatalytic fluoroalkylation by ligand-to-metal charge transfer.Front Chem. 2024 Sep 6;12:1481342. doi: 10.3389/fchem.2024.1481342. eCollection 2024. Front Chem. 2024. PMID: 39308850 Free PMC article. Review.
-
Iron(II)-Catalyzed Azidotrifluoromethylation of Olefins and N-Heterocycles for Expedient Vicinal Trifluoromethyl Amine Synthesis.ACS Catal. 2018 Jun 1;8(6):5032-5037. doi: 10.1021/acscatal.8b01253. Epub 2018 Apr 20. ACS Catal. 2018. PMID: 29938121 Free PMC article.
-
Recent Advances on the Halo- and Cyano-Trifluoromethylation of Alkenes and Alkynes.Molecules. 2021 Nov 28;26(23):7221. doi: 10.3390/molecules26237221. Molecules. 2021. PMID: 34885802 Free PMC article. Review.
-
Vicinal halo-trifluoromethylation of alkenes.RSC Adv. 2021 Apr 21;11(25):14941-14955. doi: 10.1039/d0ra06872a. eCollection 2021 Apr 21. RSC Adv. 2021. PMID: 35424045 Free PMC article. Review.
-
The dual role of thiourea in the thiotrifluoromethylation of alkenes.Chem Sci. 2017 Feb 1;8(2):1195-1199. doi: 10.1039/c6sc02790c. Epub 2016 Sep 30. Chem Sci. 2017. PMID: 28451260 Free PMC article.
References
-
- Kirsch P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications. Weinheim: Wiley-VCH; 2004.
-
- Bégué J-P, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Hoboken: Wiley-VHC; 2008.
-
- Ojima I. Medicinal Chemistry and Chemical Biology. Oxford: Wiley-Blackwell; 2009. Fluorine.
-
- Jeschke P. ChemBioChem. 2004;5:570–589. - PubMed
-
- Böhm H-J, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources