Molecular mechanisms underlying the memory-enhancing effects of estradiol
- PMID: 25960081
- PMCID: PMC4573242
- DOI: 10.1016/j.yhbeh.2015.05.001
Molecular mechanisms underlying the memory-enhancing effects of estradiol
Abstract
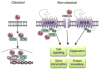
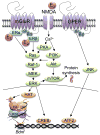
This article is part of a Special Issue "Estradiol and cognition". Since the publication of the 1998 special issue of Hormones and Behavior on estrogens and cognition, substantial progress has been made towards understanding the molecular mechanisms through which 17β-estradiol (E2) regulates hippocampal plasticity and memory. Recent research has demonstrated that rapid effects of E2 on hippocampal cell signaling, epigenetic processes, and local protein synthesis are necessary for E2 to facilitate the consolidation of object recognition and spatial memories in ovariectomized female rodents. These effects appear to be mediated by non-classical actions of the intracellular estrogen receptors ERα and ERβ, and possibly by membrane-bound ERs such as the G-protein-coupled estrogen receptor (GPER). New findings also suggest a key role of hippocampally-synthesized E2 in regulating hippocampal memory formation. The present review discusses these findings in detail and suggests avenues for future study.
Keywords: Cell signaling; DNA methylation; ERK; Epigenetic; Estrogen; Estrogen receptor; GPER; Hippocampus; Histone acetylation; mTOR.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

