APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern
- PMID: 25960448
- PMCID: PMC4637003
- DOI: 10.1016/j.jalz.2015.03.003
APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern
Abstract
Introduction: This study assessed apolipoprotein E (APOE) ε4 carrier status effects on Alzheimer's disease imaging and cerebrospinal fluid (CSF) biomarkers in cognitively normal older adults with significant memory concerns (SMC).
Methods: Cognitively normal, SMC, and early mild cognitive impairment participants from Alzheimer's Disease Neuroimaging Initiative were divided by APOE ε4 carrier status. Diagnostic and APOE effects were evaluated with emphasis on SMC. Additional analyses in SMC evaluated the effect of the interaction between APOE and [(18)F]Florbetapir amyloid positivity on CSF biomarkers.
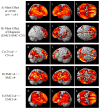
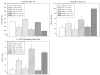
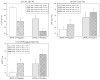
Results: SMC ε4+ showed greater amyloid deposition than SMC ε4-, but no hypometabolism or medial temporal lobe (MTL) atrophy. SMC ε4+ showed lower amyloid beta 1-42 and higher tau/p-tau than ε4-, which was most abnormal in APOE ε4+ and cerebral amyloid positive SMC.
Discussion: SMC APOE ε4+ show abnormal changes in amyloid and tau biomarkers, but no hypometabolism or MTL neurodegeneration, reflecting the at-risk nature of the SMC group and the importance of APOE in mediating this risk.
Keywords: ADNI; APOE; Alzheimer's Disease Neuroimaging Initiative; Apolipoprotein E; CSF; Cerebrospinal fluid; FDG; Neuroimaging; PET; SCD; SMC; Significant memory concern; Structural magnetic resonance imaging (MRI); Subjective cognitive decline; [(18)F]Florbetapir PET; [(18)F]Fluorodeoxyglucose.
Copyright © 2015 The Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10:e47–e92. - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 AG030514/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- R01 AG019771/AG/NIA NIH HHS/United States
- R00 LM011384/LM/NLM NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- K99 LM011384/LM/NLM NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- CAPMC/ CIHR/Canada
- UL1 TR001108/TR/NCATS NIH HHS/United States
- R01 AG19771/AG/NIA NIH HHS/United States
- K01 AG049050/AG/NIA NIH HHS/United States
- P30 AG010129/AG/NIA NIH HHS/United States
- R01 LM011360/LM/NLM NIH HHS/United States
- P30 AG10133-18S1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

