Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan
- PMID: 25960646
- PMCID: PMC4410821
- DOI: 10.2147/COPD.S56067
Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan
Abstract
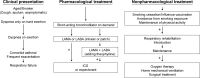
Once-daily dual-bronchodilator therapy with combined indacaterol and glycopyrronium bromide in one device (Ultibro, Breezhaler), often called QVA149, was first approved in 2013 in Japan and Europe. As of November 2014, more than 40 countries had approved this medication except for the USA. This is the first dual bronchodilator in one device. Now, the Breezhaler is the only device that can provide long-acting muscarinic antagonist (glycopyrronium bromide), long-acting beta agonist (indacaterol), and a combination of the two medications (QVA149). The choice among the three medications allows a patient to use the same inhalation device even when the regimen is changed from single-bronchodilator therapy to dual-bronchodilator therapy. In addition, the quick bronchodilation effect and once-daily administration can improve patient adherence to medical treatment for chronic obstructive pulmonary disease (COPD). To our knowledge, as of November 2014, the safety and the efficacy of QVA149 have been evaluated in 14 randomized controlled trials. The 14 trials generally showed good safety profiles, and there were better or not-inferior bronchodilator effects of QVA149 when compared with placebo, or other inhaled medication. According to the Japanese Respiratory Society guidelines, QVA149 is a combination of the two first-line bronchodilators. Our meta-analysis indicated that QVA149 is superior to the salmeterol-fluticasone combination to treat COPD in respect of the frequency of adverse effects, exacerbation, pneumonia, and improvement of trough forced expiratory volume in 1 second (FEV1). Thus, we believe that QVA149 can be a key medication for COPD treatments.
Keywords: bronchodilator agents; delivery of health care; dry powder inhalers; guideline; meta-analysis; muscarinic antagonists.
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of COPD. 2014. [Accessed October 20, 2014]. Available from: http://www.goldcopd.org/
-
- Donohue JF, Fogarty C, Lötvall J, et al. INHANCE Study Investigators Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

