Rituximab leads to long remissions in patients with chronic immune thrombocytopenia
- PMID: 25960836
- PMCID: PMC4412453
- DOI: 10.5001/omj.2015.24
Rituximab leads to long remissions in patients with chronic immune thrombocytopenia
Abstract
Objectives: To assess the response rate and duration of response in patients with chronic immune thrombocytopenia (ITP) receiving rituximab.
Methods: We retrospectively analyzed 32 consecutive patients with chronic ITP who were treated in two tertiary centers in Oman. Response assessment was based on the American Society of Hematology criteria.
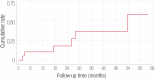
Results: Nineteen patients (59%) had an initial response. However, six of the 19 patients lost their response leaving 13 patients with long-lasting remissions. The median age at diagnosis was 25 years (range 14-58). The median time from diagnosis to rituximab therapy was 21 months. The median follow-up after starting rituximab was 26 months. The overall cumulative response rate was 59% (complete response 44%, partial response 15%) and the median time to respond was 30 days with a response rate of 44% at four weeks. In all responders, the cumulative rate of loss of response was 32% with a median time to lose response of 54 months.
Conclusions: The use of rituximab in ITP achieves high response rate and long remission duration. Our study was limited by the small sample size and further larger prospective studies are recommended.
Keywords: Blood Platelets; Purpura, Thrombocytopenic, Idiopathic; Rituximab.
Figures
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. . Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009. Mar;113(11):2386-2393. http://www.ncbi.nlm.nih.gov/pubmed/19005182. Accessed 27 May 2014. Internet. 10.1182/blood-2008-07-162503 - DOI - PubMed
-
- Stasi R, Evangelista ML, Stipa E, Buccisano F, Venditti A, Amadori S. Idiopathic thrombocytopenic purpura: current concepts in pathophysiology and management. Thromb Haemost 2008. Jan;99(1):4-13. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources