A beginning of the end: new insights into the functional organization of telomeres
- PMID: 25961132
- PMCID: PMC4615733
- DOI: 10.1080/19491034.2015.1048407
A beginning of the end: new insights into the functional organization of telomeres
Abstract
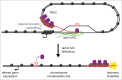
Ever since the first demonstration of their repetitive sequence and unique replication pathway, telomeres have beguiled researchers with how they function in protecting chromosome ends. Of course much has been learned over the years, and we now appreciate that telomeres are comprised of the multimeric protein/DNA shelterin complex and that the formation of t-loops provides protection from DNA damage machinery. Deriving their name from D-loops, t-loops are generated by the insertion of the 3' overhang into telomeric repeats facilitated by the binding of TRF2. Recent studies have uncovered novel forms of chromosome end-structure that may implicate telomere organization in cellular processes beyond its essential role in telomere protection and homeostasis. In particular, we have recently described that t-loops form in a TRF2-dependent manner at interstitial telomere repeat sequences, which we termed interstitial telomere loops (ITLs). These structures are also dependent on association of lamin A/C, a canonical component of the nucleoskeleton that is mutated in myriad human diseases, including human segmental progeroid syndromes. Since ITLs are associated with telomere stability and require functional lamin A/C, our study suggests a mechanistic link between cellular aging (replicative senescence induced by telomere shortening) and organismal aging (modeled by Hutchinson Gilford Progeria Syndrome). Here we speculate on other potential ramifications of ITL formation, from gene expression to genome stability to chromosome structure.
Keywords: aging; chromosome looping; chromosome structure; genome stability; nuclear lamina; telomere.
Figures
References
-
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19:2100-10; PMID:16166375; http://dx.doi.org/10.1101/gad.1346005. - DOI - PubMed
-
- van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature 1997; 385:740-3; PMID:9034193; http://dx.doi.org/10.1038/385740a0. - DOI - PubMed
-
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 2000; 20:1659-68; PMID:10669743; http://dx.doi.org/10.1128/MCB.20.5.1659-1668.2000. - DOI - PMC - PubMed
-
- Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science 2002; 295:2446-9; http://dx.doi.org/10.1126/science.1069523. - DOI - PubMed
-
- Karlseder J. Telomere repeat binding factors: keeping the ends in check. Cancer Lett 2003; 194:189-97; PMID:12757977; http://dx.doi.org/10.1016/S0304-3835(02)00706-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
