A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults
- PMID: 25961283
- PMCID: PMC4427332
- DOI: 10.1371/journal.pone.0125954
A Phase I Double Blind, Placebo-Controlled, Randomized Study of the Safety and Immunogenicity of an Adjuvanted HIV-1 Gag-Pol-Nef Fusion Protein and Adenovirus 35 Gag-RT-Int-Nef Vaccine in Healthy HIV-Uninfected African Adults
Abstract
Background: Sequential prime-boost or co-administration of HIV vaccine candidates based on an adjuvanted clade B p24, RT, Nef, p17 fusion protein (F4/AS01) plus a non-replicating adenovirus 35 expressing clade A Gag, RT, Int and Nef (Ad35-GRIN) may lead to a unique immune profile, inducing both strong T-cell and antibody responses.
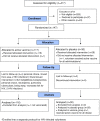
Methods: In a phase 1, double-blind, placebo-controlled trial, 146 healthy adult volunteers were randomized to one of four regimens: heterologous prime-boost with two doses of F4/AS01E or F4/AS01B followed by Ad35-GRIN; Ad35-GRIN followed by two doses of F4/AS01B; or three co-administrations of Ad35-GRIN and F4/AS01B. T cell and antibody responses were measured.
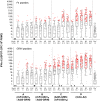
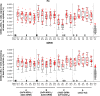
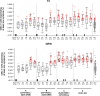
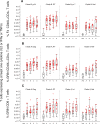
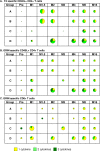
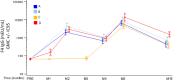
Results: The vaccines were generally well-tolerated, and did not cause serious adverse events. The response rate, by IFN-γ ELISPOT, was greater when Ad35-GRIN was the priming vaccine and in the co-administration groups. F4/AS01 induced CD4+ T-cells expressing primarily CD40L and IL2 +/- TNF-α, while Ad35-GRIN induced predominantly CD8+ T-cells expressing IFN-γ +/- IL2 or TNF-α. Viral inhibition was induced after Ad35-GRIN vaccination, regardless of the regimen. Strong F4-specific antibody responses were induced. Immune responses persisted at least a year after the last vaccination. The complementary response profiles, characteristic of each vaccine, were both expressed after co-administration.
Conclusion: Co-administration of an adjuvanted protein and an adenovirus vector showed an acceptable safety and reactogenicity profile and resulted in strong, multifunctional and complementary HIV-specific immune responses.
Trial registration: ClinicalTrials.gov NCT01264445.
Conflict of interest statement
Figures


References
-
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6776–81. 10.1073/pnas.0611244104 - DOI - PMC - PubMed
-
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–60. . - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

