Chaperone-Mediated Autophagy Targets IFNAR1 for Lysosomal Degradation in Free Fatty Acid Treated HCV Cell Culture
- PMID: 25961570
- PMCID: PMC4427131
- DOI: 10.1371/journal.pone.0125962
Chaperone-Mediated Autophagy Targets IFNAR1 for Lysosomal Degradation in Free Fatty Acid Treated HCV Cell Culture
Expression of concern in
-
Expression of Concern: Chaperone-Mediated Autophagy Targets IFNAR1 for Lysosomal Degradation in Free Fatty Acid Treated HCV Cell Culture.PLoS One. 2025 Feb 7;20(2):e0319262. doi: 10.1371/journal.pone.0319262. eCollection 2025. PLoS One. 2025. PMID: 39919083 Free PMC article. No abstract available.
Abstract
Background: Hepatic steatosis is a risk factor for both liver disease progression and an impaired response to interferon alpha (IFN-α)-based combination therapy in chronic hepatitis C virus (HCV) infection. Previously, we reported that free fatty acid (FFA)-treated HCV cell culture induces hepatocellular steatosis and impairs the expression of interferon alpha receptor-1 (IFNAR1), which is why the antiviral activity of IFN-α against HCV is impaired.
Aim: To investigate the molecular mechanism by which IFNAR1 expression is impaired in HCV cell culture with or without free fatty acid-treatment.
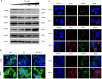
Method: HCV-infected Huh 7.5 cells were cultured with or without a mixture of saturated (palmitate) and unsaturated (oleate) long-chain free fatty acids (FFA). Intracytoplasmic fat accumulation in HCV-infected culture was visualized by oil red staining. Clearance of HCV in FFA cell culture treated with type I IFN (IFN-α) and Type III IFN (IFN-λ) was determined by Renilla luciferase activity, and the expression of HCV core was determined by immunostaining. Activation of Jak-Stat signaling in the FFA-treated HCV culture by IFN-α alone and IFN-λ alone was examined by Western blot analysis and confocal microscopy. Lysosomal degradation of IFNAR1 by chaperone-mediated autophagy (CMA) in the FFA-treated HCV cell culture model was investigated.
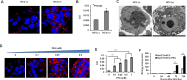
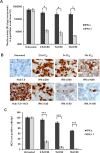
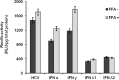
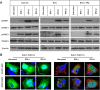
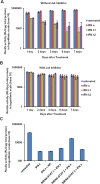
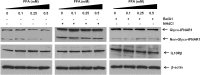
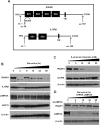
Results: FFA treatment induced dose-dependent hepatocellular steatosis and lipid droplet accumulation in HCV-infected Huh-7.5 cells. FFA treatment of infected culture increased HCV replication in a concentration-dependent manner. Intracellular lipid accumulation led to reduced Stat phosphorylation and nuclear translocation, causing an impaired IFN-α antiviral response and HCV clearance. Type III IFN (IFN-λ), which binds to a separate receptor, induces Stat phosphorylation, and nuclear translocation as well as antiviral clearance in FFA-treated HCV cell culture. We show here that the HCV-induced autophagy response is increased in FFA-treated cell culture. Pharmacological inhibitors of lysosomal degradation, such as ammonium chloride and bafilomycin, prevented IFNAR1 degradation in FFA-treated HCV cell culture. Activators of chaperone-mediated autophagy, including 6-aminonicotinamide and nutrient starvation, decreased IFNAR1 levels in Huh-7.5 cells. Co-immunoprecipitation, colocalization and siRNA knockdown experiments revealed that IFNAR1 but not IFNLR1 interacts with HSC70 and LAMP2A, which are core components of chaperone-mediated autophagy (CMA).
Conclusion: Our study presents evidence indicating that chaperone-mediated autophagy targets IFNAR1 degradation in the lysosome in FFA-treated HCV cell culture. These results provide a mechanism for why HCV induced autophagy response selectively degrades type I but not the type III IFNAR1.
Conflict of interest statement
Figures


Similar articles
-
Hepatitis C Virus Infection Induces Autophagy as a Prosurvival Mechanism to Alleviate Hepatic ER-Stress Response.Viruses. 2016 May 23;8(5):150. doi: 10.3390/v8050150. Viruses. 2016. PMID: 27223299 Free PMC article. Review.
-
Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture.Virol J. 2012 Aug 3;9:143. doi: 10.1186/1743-422X-9-143. Virol J. 2012. PMID: 22863531 Free PMC article.
-
HCV infection selectively impairs type I but not type III IFN signaling.Am J Pathol. 2014 Jan;184(1):214-29. doi: 10.1016/j.ajpath.2013.10.005. Epub 2013 Nov 9. Am J Pathol. 2014. PMID: 24215913 Free PMC article.
-
Impaired expression of type I and type II interferon receptors in HCV-associated chronic liver disease and liver cirrhosis.PLoS One. 2014 Sep 29;9(9):e108616. doi: 10.1371/journal.pone.0108616. eCollection 2014. PLoS One. 2014. PMID: 25265476 Free PMC article.
-
Chaperone-Mediated Autophagy in the Liver: Good or Bad?Cells. 2019 Oct 24;8(11):1308. doi: 10.3390/cells8111308. Cells. 2019. PMID: 31652893 Free PMC article. Review.
Cited by
-
Diverse Functions of Autophagy in Liver Physiology and Liver Diseases.Int J Mol Sci. 2019 Jan 13;20(2):300. doi: 10.3390/ijms20020300. Int J Mol Sci. 2019. PMID: 30642133 Free PMC article. Review.
-
Integrated stress response in hepatitis C promotes Nrf2-related chaperone-mediated autophagy: A novel mechanism for host-microbe survival and HCC development in liver cirrhosis.Semin Cell Dev Biol. 2020 May;101:20-35. doi: 10.1016/j.semcdb.2019.07.015. Epub 2019 Aug 8. Semin Cell Dev Biol. 2020. PMID: 31386899 Free PMC article. Review.
-
Interactions of Autophagy and the Immune System in Health and Diseases.Autophagy Rep. 2022;1(1):438-515. doi: 10.1080/27694127.2022.2119743. Epub 2022 Oct 5. Autophagy Rep. 2022. PMID: 37425656 Free PMC article.
-
Hepatitis C Virus Infection Induces Autophagy as a Prosurvival Mechanism to Alleviate Hepatic ER-Stress Response.Viruses. 2016 May 23;8(5):150. doi: 10.3390/v8050150. Viruses. 2016. PMID: 27223299 Free PMC article. Review.
-
Regulation of Autophagy in Cells Infected With Oncogenic Human Viruses and Its Impact on Cancer Development.Front Cell Dev Biol. 2020 Feb 28;8:47. doi: 10.3389/fcell.2020.00047. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32181249 Free PMC article. Review.
References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2006; 5:558–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

