Drug Dosing and Pharmacokinetics in Children With Obesity: A Systematic Review
- PMID: 25961828
- PMCID: PMC4494887
- DOI: 10.1001/jamapediatrics.2015.132
Drug Dosing and Pharmacokinetics in Children With Obesity: A Systematic Review
Erratum in
- JAMA Pediatr. 2015 Dec;169(12):1179. Becker, Kristian [corrected to Becker, Kristian C]
Abstract
Importance: Obesity affects nearly one-sixth of US children and results in alterations to body composition and physiology that can affect drug disposition, possibly leading to therapeutic failure or toxic side effects. The depth of available literature regarding obesity's effect on drug safety, pharmacokinetics, and dosing in obese children is unknown.
Objective: To perform a systematic literature review describing the current evidence of the effect of obesity on drug disposition in children.
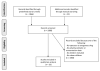
Evidence review: We searched the MEDLINE, Cochrane, and EMBASE databases (January 1, 1970-December 31, 2012) and included studies if they contained data on drug clearance, volume of distribution, or drug concentration in obese children (aged ≤18 years). We compared exposure and weight-normalized volume of distribution and clearance between obese and nonobese children. We explored the association between drug physicochemical properties and clearance and volume of distribution.
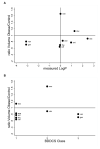
Findings: Twenty studies met the inclusion criteria and contained pharmacokinetic data for 21 drugs. The median number of obese children studied per drug was 10 (range, 1-112) and ages ranged from newborn to 29 years (1 study described pharmacokinetics in children and adults together). Dosing schema varied and were either a fixed dose (6 [29%]) or based on body weight (10 [48%]) and body surface area (4 [19%]). Clinically significant pharmacokinetic alterations were observed in obese children for 65% (11 of 17) of the studied drugs. Pharmacokinetic alterations resulted in substantial differences in exposure between obese and nonobese children for 38% (5 of 13) of the drugs. We found no association between drug lipophilicity or Biopharmaceutical Drug Disposition Classification System class and changes in volume of distribution or clearance due to obesity.
Conclusions and relevance: Consensus is lacking on the most appropriate weight-based dosing strategy for obese children. Prospective pharmacokinetic trials in obese children are needed to ensure therapeutic efficacy and enhance drug safety.
Figures

Comment in
-
Children With Obesity: How Are They Different?JAMA Pediatr. 2015 Jul;169(7):626-8. doi: 10.1001/jamapediatrics.2015.0444. JAMA Pediatr. 2015. PMID: 25961595 Free PMC article. No abstract available.
References
-
- Hampl SE, Carroll CA, Simon SD, Sharma V. Resource utilization and expenditures for overweight and obese children. Arch Pediatr Adolesc Med. 2007;161(1):11–14. - PubMed
-
- Hering E, Pritsker I, Gonchar L, Pillar G. Obesity in children is associated with increased health care use. Clin Pediatr. 2009;48(8):812–818. - PubMed
Publication types
MeSH terms
Grants and funding
- HHSN275201000001Z/HD/NICHD NIH HHS/United States
- 1U01FD004858-01/FD/FDA HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- UL1TR001117/TR/NCATS NIH HHS/United States
- KL2 TR001115/TR/NCATS NIH HHS/United States
- K23 HD064814/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- K23 HD075891/HD/NICHD NIH HHS/United States
- 1T32GM086330-01A1/GM/NIGMS NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- 1K23HD064814/HD/NICHD NIH HHS/United States
- T32GM086330/GM/NIGMS NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- U01 FD004858/FD/FDA HHS/United States
- T32 GM086330/GM/NIGMS NIH HHS/United States
- 1K24HD058735-05/HD/NICHD NIH HHS/United States
- 1R01HD057956-05/HD/NICHD NIH HHS/United States
- HHSN275201000001G/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

