The Repellent DEET Potentiates Carbamate Effects via Insect Muscarinic Receptor Interactions: An Alternative Strategy to Control Insect Vector-Borne Diseases
- PMID: 25961834
- PMCID: PMC4427492
- DOI: 10.1371/journal.pone.0126406
The Repellent DEET Potentiates Carbamate Effects via Insect Muscarinic Receptor Interactions: An Alternative Strategy to Control Insect Vector-Borne Diseases
Abstract
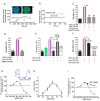
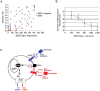
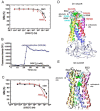
Insect vector-borne diseases remain one of the principal causes of human mortality. In addition to conventional measures of insect control, repellents continue to be the mainstay for personal protection. Because of the increasing pyrethroid-resistant mosquito populations, alternative strategies to reconstitute pyrethroid repellency and knock-down effects have been proposed by mixing the repellent DEET (N,N-Diethyl-3-methylbenzamide) with non-pyrethroid insecticide to better control resistant insect vector-borne diseases. By using electrophysiological, biochemichal, in vivo toxicological techniques together with calcium imaging, binding studies and in silico docking, we have shown that DEET, at low concentrations, interacts with high affinity with insect M1/M3 mAChR allosteric site potentiating agonist effects on mAChRs coupled to phospholipase C second messenger pathway. This increases the anticholinesterase activity of the carbamate propoxur through calcium-dependent regulation of acetylcholinesterase. At high concentrations, DEET interacts with low affinity on distinct M1/M3 mAChR site, counteracting the potentiation. Similar dose-dependent dual effects of DEET have also been observed at synaptic mAChR level. Additionally, binding and in silico docking studies performed on human M1 and M3 mAChR subtypes indicate that DEET only displays a low affinity antagonist profile on these M1/M3 mAChRs. These results reveal a selective high affinity positive allosteric site for DEET in insect mAChRs. Finally, bioassays conducted on Aedes aegypti confirm the synergistic interaction between DEET and propoxur observed in vitro, resulting in a higher mortality of mosquitoes. Our findings reveal an unusual allosterically potentiating action of the repellent DEET, which involves a selective site in insect. These results open exciting research areas in public health particularly in the control of the pyrethroid-resistant insect-vector borne diseases. Mixing low doses of DEET and a non-pyrethroid insecticide will lead to improvement in the efficiency treatments thus reducing both the concentration of active ingredients and side effects for non-target organisms. The discovery of this insect specific site may pave the way for the development of new strategies essential in the management of chemical use against resistant mosquitoes.
Conflict of interest statement
Figures


References
-
- Dickens JC, Bohbot JD (2013) Mini review: Mode of action of mosquito repellents. Pest Biochem Physiol 106, 149–155.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

