Cellular promyelocytic leukemia protein is an important dengue virus restriction factor
- PMID: 25962098
- PMCID: PMC4427460
- DOI: 10.1371/journal.pone.0125690
Cellular promyelocytic leukemia protein is an important dengue virus restriction factor
Erratum in
-
Correction: Cellular Promyelocytic Leukemia Protein Is an Important Dengue Virus Restriction Factor.PLoS One. 2015 Aug 27;10(8):e0137040. doi: 10.1371/journal.pone.0137040. eCollection 2015. PLoS One. 2015. PMID: 26313937 Free PMC article. No abstract available.
Abstract
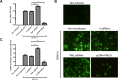
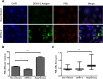
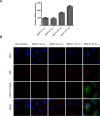
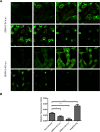
The intrinsic antiviral defense is based on cellular restriction factors that are constitutively expressed and, thus, active even before a pathogen enters the cell. The promyelocytic leukemia (PML) nuclear bodies (NBs) are discrete nuclear foci that contain several cellular proteins involved in intrinsic antiviral responses against a number of viruses. Accumulating reports have shown the importance of PML as a DNA virus restriction factor and how these pathogens evade this antiviral activity. However, very little information is available regarding the antiviral role of PML against RNA viruses. Dengue virus (DENV) is an RNA emerging mosquito-borne human pathogen affecting millions of individuals each year by causing severe and potentially fatal syndromes. Since no licensed antiviral drug against DENV infection is currently available, it is of great importance to understand the factors mediating intrinsic immunity that may lead to the development of new pharmacological agents that can boost their potency and thereby lead to treatments for this viral disease. In the present study, we investigated the in vitro antiviral role of PML in DENV-2 A549 infected cells.
Conflict of interest statement
Figures

References
-
- Maul GG, Negorev D, Bell P, Ishov AM (2000) Review: properties and assembly mechanisms of ND10, PML bodies, or PODs. J Struct Biol 129: 278–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

