Therapeutic Basis of Clinical Pain Modulation
- PMID: 25962969
- PMCID: PMC4641846
- DOI: 10.1111/cts.12282
Therapeutic Basis of Clinical Pain Modulation
Abstract
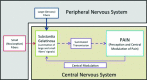
Pain is a hallmark of almost all bodily ailments and can be modulated by agents, including analgesics and anesthetics that suppress pain signals in the central nervous system. Defects in the modulatory systems, including the endogenous pain-inhibitory pathways, are a major factor in the initiation and chronicity of pain. Thus, pain modulation is particularly applicable to the practice of medicine. This review summarizes the existing literature on pain modulation. Here, we critically reviewed the literature from PubMed on pain modulation published primarily within the past 5 years in high impact journals. Specifically, we have discussed important anatomical landmarks of pain modulation and outlined the endogenous networks and underlying mechanisms of clinically relevant pain modulatory methods. The Gate Control Theory is briefly presented with discussion on the capacity of pain modulation to cause both hyper- and hypoalgesia. An emphasis has been given to highlight key areas in pain research that, because of unanswered questions or therapeutic potential, merit additional scientific scrutiny. The information presented in this paper would be helpful in developing novel therapies, metrics, and interventions for improved patient management.
Keywords: Gate Control Theory; cannabinoids; electroanalgesia; inhibitory amino acids; opioids; pain modulation; periaqueductal gray; rostral ventromedial medulla.
© 2015 Wiley Periodicals, Inc.
Figures



References
-
- Busch‐Dienstfertig M, Stein C. Opioid receptors and opioid peptide‐producing leukocytes in inflammatory pain—basic and therapeutic aspects. Brain Behav Immun. 2010; 24(5): 683–694. - PubMed
-
- Watanabi C. Mechanism of spinal pain transmission and its regulation. Yakugaku Zasshi. 2014; 134(12): 1301–1307. - PubMed
-
- He Z, Guo Q, Xiao M, He C, Zou W. Intrathecal lentivirus‐mediated transfer of interleukin‐10 attenuates chronic constriction injury‐induced neuropathic pain through modulation of spinal high‐mobility group box 1 in rats. Pain Physician. 2013; 16(5): E615–625. - PubMed
-
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150(3699): 971–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

