External stimulation-controllable heat-storage ceramics
- PMID: 25962982
- PMCID: PMC4432584
- DOI: 10.1038/ncomms8037
External stimulation-controllable heat-storage ceramics
Abstract
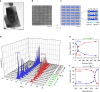
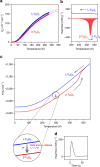
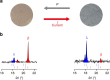
Commonly available heat-storage materials cannot usually store the energy for a prolonged period. If a solid material could conserve the accumulated thermal energy, then its heat-storage application potential is considerably widened. Here we report a phase transition material that can conserve the latent heat energy in a wide temperature range, T<530 K and release the heat energy on the application of pressure. This material is stripe-type lambda-trititanium pentoxide, λ-Ti3O5, which exhibits a solid-solid phase transition to beta-trititanium pentoxide, β-Ti3O5. The pressure for conversion is extremely small, only 600 bar (60 MPa) at ambient temperature, and the accumulated heat energy is surprisingly large (230 kJ L(-1)). Conversely, the pressure-produced beta-trititanium pentoxide transforms to lambda-trititanium pentoxide by heat, light or electric current. That is, the present system exhibits pressure-and-heat, pressure-and-light and pressure-and-current reversible phase transitions. The material may be useful for heat storage, as well as in sensor and switching memory device applications.
Figures


References
-
- Nayak A. P. et al.. Pressure-induced semiconducting to metallic transition in multilayered molybdenum disulphide. Nat. Commun. 5, 3731 (2014). - PubMed
-
- Takabayashi Y. et al.. The disorder-free non-BCS superconductor Cs3C60 emerges from an antiferromagnetic insulator parent state. Science 323, 1585–1590 (2009). - PubMed
-
- Avdeev M. et al.. Pressure-induced ferroelectric to antiferroelectric phase transition in Pb0.99(Zr0.95Ti0.05)0.98Nb0.02O3. Phys. Rev. B 73, 064105/1–14 (2006).
-
- Kolobov A. V. et al.. Understanding the phase-change mechanism of rewritable optical media. Nat. Mater. 3, 703–708 (2004). - PubMed
-
- Wuttig M. & Yamada N. Phase-change materials for rewriteable data storage. Nat. Mater. 6, 824–832 (2007). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

