S-phase duration is the main target of cell cycle regulation in neural progenitors of developing ferret neocortex
- PMID: 25963823
- PMCID: PMC5008145
- DOI: 10.1002/cne.23801
S-phase duration is the main target of cell cycle regulation in neural progenitors of developing ferret neocortex
Abstract
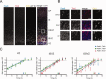
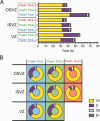
The evolutionary expansion of the neocortex primarily reflects increases in abundance and proliferative capacity of cortical progenitors and in the length of the neurogenic period during development. Cell cycle parameters of neocortical progenitors are an important determinant of cortical development. The ferret (Mustela putorius furo), a gyrencephalic mammal, has gained increasing importance as a model for studying corticogenesis. Here, we have studied the abundance, proliferation, and cell cycle parameters of different neural progenitor types, defined by their differential expression of the transcription factors Pax6 and Tbr2, in the various germinal zones of developing ferret neocortex. We focused our analyses on postnatal day 1, a late stage of cortical neurogenesis when upper-layer neurons are produced. Based on cumulative 5-ethynyl-2'-deoxyuridine (EdU) labeling as well as Ki67 and proliferating cell nuclear antigen (PCNA) immunofluorescence, we determined the duration of the various cell cycle phases of the different neocortical progenitor subpopulations. Ferret neocortical progenitors were found to exhibit longer cell cycles than those of rodents and little variation in the duration of G1 among distinct progenitor types, also in contrast to rodents. Remarkably, the main difference in cell cycle parameters among the various progenitor types was the duration of S-phase, which became shorter as progenitors progressively changed transcription factor expression from patterns characteristic of self-renewal to those of neuron production. Hence, S-phase duration emerges as major target of cell cycle regulation in cortical progenitors of this gyrencephalic mammal.
Keywords: AB_10569705; AB_10615604; AB_2142367; AB_2295065; AB_778267; AB_95106; RRIDs: AB_2313780; SciRes_000137; cortical development; evolution; gyrencephaly; neurogenesis; nif-0000-30467.
© 2015 The Authors The Journal of Comparative Neurology Published by Wiley Periodicals, Inc.
Figures




References
-
- Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin‐Ménard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, Dehay C. 2013. Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 80:442–457. - PubMed
-
- Borrell V, Calegari F. 2014. Mechanisms of brain evolution: regulation of neural progenitor cell diversity and cell cycle length. Neurosci Res 86:14–24. - PubMed
-
- Borrell V, Reillo I. 2012. Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev Neurobiol 72:955–971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

